- Title
-
Sid4: A secreted vertebrate immunoglobulin protein with roles in zebrafish embryogenesis
- Authors
- diIorio, P.J., Runko, A., Farrell, C.A., and Roy, N.
- Source
- Full text @ Dev. Biol.
|
sid4 expression during normal zebrafish development. (A) Embryos hybridized to sid4 antisense probe exhibited ubiquitous expression throughout early embryogenesis. (B) No signal was seen in embryos hybridized to the sense probe. (C) sid4 is expressed throughout the blastoderm at 4 hpf and (D) in the hypoblast (h), epiblast (ep), and ventral blastomeres (v) of gastrulating embryos. (E) sid4 is detected in the eye primordia (e), somites (s) and central nervous system of 16 hpf embryos. (F) sid4 is expressed in the forebrain (fb), hindbrain (hb), eyes, and pharyngeal arch region (p) of 24 hpf embryos. (G) dlx-2 expression is more restricted than sid4 within the pharyngeal arch. (H) The inner cell mass (icm) and (I) hatching gland (hg) of 24 hpf embryos also express sid4. (J) sid4 is absent from the 28-hpf hindbrain (red arrow) but prominently expressed in ventricle IV (IV), telencephalon (t), and diencephalon (d). (K) Scattered blood cells (bc) and (L) fin buds (fb) express sid4 at 40 hpf. (M) By 48 hpf, sid4 is restricted to the liver, where it remains through 7 days of development (N). Lateral views in A–D; H, K. Dorsal views in E–G; J–N. Anterodorsal view in I. Embryonic stages are indicated at the lower left of each panel. |
|
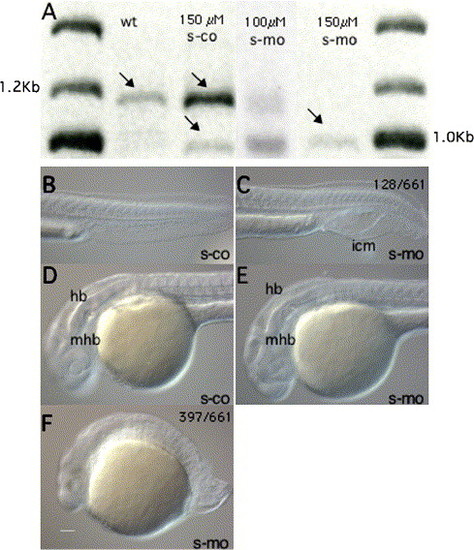
Morpholino injection inhibits correct sid4 mRNA splicing and disrupts development of mesoderm and neuroectoderm. (A) RT-PCR demonstrates dose-response s-mo-specific deletion of sid4 exon 5. Treatments are given at the top of each lane. Arrows indicate amplification products that were cloned and sequenced. (B–G) Lateral views of live 24 hpf zebrafish embryos injected with 150 μM s-mo and s-co (B–F) and t-mo (G). (B) Tail mesoderm is unaffected by s-co. (C) Mildly affected morphant embryos exhibit expanded inner cell mass and defects in caudal somite morphology. Anterior somites appear normal. (D) Brain regionalization is normal in s-co and (E) mildly affected morphants. (F) In the majority of s-mo morphants, somites are rounded, tails severely truncated and brain structures indistinct. Magnification is the same in all panels. Anterior is to the left. hb, hindbrain; icm, inner cell mass; mhb, midbrain–hindbrain boundary. EXPRESSION / LABELING:
|
|
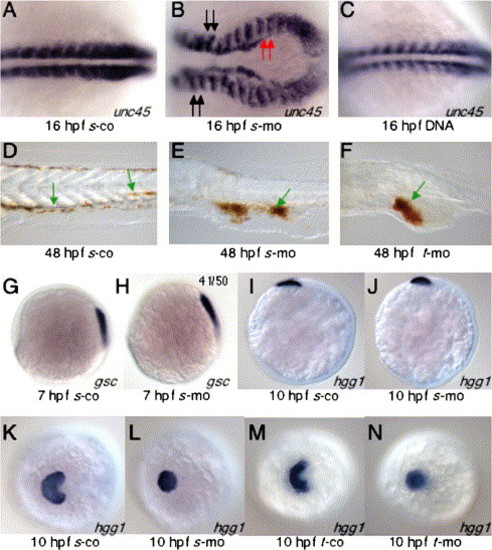
sid4 morpholinos cause morphogenetic defects and increased apoptosis. (A) s-mo and (B) t-mo produce similar epiboly defects. In these embryos, the blastoderm margin appears to constrict before the blastoderm completely covers the yolk. (C) Actively gastrulating control and (D) morphant embryos (90% epiboly) do not exhibit apoptosis. (E) Flh is appropriately expressed in the neural plate and polster of 10 hpf s-co and (F) s-mo embryos. The number of anterior flh+cells appears reduced in the morphants. (G) Flh-expressing cells extend into the tail bud (blue arrow) of s-co embryos. (H) Yolk extrusions (green arrow) inhibit normal tail bud formation and patterning of caudal flh+cells. (I) Apoptosis increases in the caudal mesoderm of 10 hpf morphants. (J) Caudal flh-expressing cells (black arrows) converge normally and extend to form a narrow, compact notochord. (K) Convergence is impaired in morphant embryos and the notochord field is much broader than controls. (L) In many cases, the distribution of apoptotic cells in 10 hpf morphants was similar to flh expression patterns. Ratios of embryos exhibiting a given phenotype are in upper right. Lateral views in A–D; G–I. Dorsal views in E, F, J–L. Embryonic stages and treatments are indicated below each panel. In situ probes and TUNEL are indicated in lower right. EXPRESSION / LABELING:
|
|
Sid4 function during development of ventral and prechordal mesoderm. (A) In s-co embryos, somitic mesoderm develops normally. (B) Somite shape and convergence of caudal somitic mesoderm are disrupted in s-mo embryos. Somites anterior (black arrows) to and involved in (red arrows) the convergence defect are similarly malformed. (C) Somite development was normal in embryos injected with 25 pg/nl of sid4 expression DNA. (D–F) Red blood cells stained with o-dianisidine. (D) Red blood cells can be seen within the vasculature of 24 hpf control embryos (green arrows). (E) In s-mo and (F) t-mo embryos, isolated red blood cells are seen in the proximity of blood vessels (green arrows), but the vast majority remain clumped within the abnormally expanded inner cell mass. (G) Anterior migration of gsc-expressing cells was similar in 7 hpf s-co and (H) s-mo embryos. (I) The anterior position of hgg1 hybridization beneath the eye primordium of 10 hpf s-co and (J) s-mo embryos was also normal. (K) Lateral migration of hatching gland cells is normal in s-co and (M) t-co morphants. (L) In both 10 hpf s-mo and (N) t-mo morphants, hatching gland cells do not spread but remain in the midline. Embryonic stages and treatment are indicated below each panel. In situ probes are indicated at the lower right of each panel. Anterior is to the left. A–J are lateral views. K–N are anterodorsal views. EXPRESSION / LABELING:
|
|
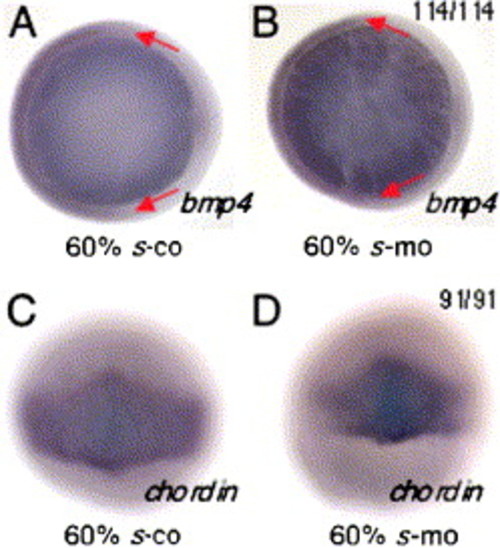
Dorsal and ventral tissues of the zebrafish gastrula are normally patterned in sid4 morphants. (A) The normal, dorsal margin of bmp4 expression is unchanged in (B) morphants. (C) No alterations of normal chordin expression were seen in (D) morphants. The light blue staining seen in C and D is a property of the BMP Purple substrate (Roche). Animal pole views in A, B; dorsal views in C, D. Embryonic stage is indicated below each panel. In situ probe is in lower right. Ratios of embryos exhibiting the phenotype are in the upper right. EXPRESSION / LABELING:
|
|
sid4 s-mo causes defects in local patterning of branchiomotor neurons and cranial neural crest development without altering early anteroposterior patterning of the brain. (A) The pattern of pax-2 expression is normal in eye primordia, midbrain hindbrain boundary, and otic vesicles of both control and (B) morphant embryos, although the number of pax-2 cells within each expression domain of morphants appears reduced. (C–F) The AP pattern of krox20 expression in rhombomeres 3 and 5 and in migrating neural crest (white arrow) is unaffected in controls (C) and morphants (D–F). (E, F) Rhombomeres are broadened and boundaries more diffuse in morphants. (G) Branchiomotor neuron patterning is normal in control embryos but increasingly disorganized in (H) mildly affected and (I) caudally truncated embryos. (J) Normal migration of pigmented, non-retinal epithelium over the eyes of 48 hpf control embryos. (K) Pigment cells are concentrated in the dorsal portion of the eye in morphants (red arrow) and fail to migrate ventrally. (L) Normal forebrain expression of dlx-2 in 24 hpf control embryos. (M) Diencephalic, dlx-2 expressing neural crest cells are reduced or absent in morphants. This phenotype does not recover in 31 hpf embryos (insets in L, M). Dorsal views in A–I. Lateral views in J–M. Anterior is to the left in all panels. Embryos in J and K were fixed and cleared in glycerol. The right eye was removed and the left eye shown in both panels. d, diencephalon; e, eye; mhb, midbrain hindbrain boundary; ov, otic vesicle; t, telencephalon. In situ probes are indicated in the lower right of each panel. EXPRESSION / LABELING:
|
|
Sid4 protein synthesis in embryos injected with mRNA and DNA. (A) Protein extracts from 12 hpf uninjected controls (lane 1), and those injected with 500 pg/nl sid4 mRNA (lane 2) exhibit similar amounts of Sid4 protein (red arrow). Robust Sid4 protein synthesis can be seen in extracts from embryos injected with 25 pg/nl pCS2sid4 DNA (Lane 3, black arrow). This new protein migrates with a mass of ∼38 kDa, the predicted size of Sid4 after signal peptide cleavage. (B) In vitro synthesized sid4 mRNA retains its integrity during microinjection. Approximately 2 µg of pre-injection (lane 1) and post-injection (lane 2) mRNA was separated on standard 0.8% agarose gels and stained with ethidium bromide. |
Reprinted from Developmental Biology, 282(1), diIorio, P.J., Runko, A., Farrell, C.A., and Roy, N., Sid4: A secreted vertebrate immunoglobulin protein with roles in zebrafish embryogenesis, 55-69, Copyright (2005) with permission from Elsevier. Full text @ Dev. Biol.