- Title
-
Protein Phosphatase 4 Cooperates with Smads to Promote BMP Signaling in Dorsoventral Patterning of Zebrafish Embryos
- Authors
- Jia, S., Dai, F., Wu, D., Lin, X., Xing, C., Xue, Y., Wang, Y., Xiao, M., Wu, W., Feng, X.H., and Meng, A.
- Source
- Full text @ Dev. Cell
|
ppp4ca and ppp4cb Spatiotemporal Expression Patterns and Their Functions on Dorsoventral Patterning in Zebrafish Embryos (A) Spatiotemporal expression pattern of ppp4ca and ppp4cb mRNA in zebrafish embryo, detected by whole-mount in situ hybridization, at indicated stages. Embryos in the first four columns were lateral views with the animal pole oriented to the top, whereas the others were oriented with anterior to the left. (B) Distribution of endogenous Ppp4c protein in zebrafish embryo at the shield stage, visualized by indirect immunofluorescence (green). The nuclei (blue) were stained with DAPI. Left: 3D reconstruction of stained embryos viewed from the animal pole by Zeiss ZEN 2009 software; right: subcellular location of Ppp4c at a higher magnification. (C) Morphological changes in ppp4ca (p4ca) and ppp4cb (p4cb) morphants at 24 hpf. Of note, p4ca or p4cb morphants exhibited variant necrosis, an off-target effect that was alleviated by coinjection of p53-MO. aMO, ppp4ca-MO; bMO, ppp4cb-MO. (D) Embryonic dorsalization induced by knockdown of ppp4ca/b was confirmed by examining several dorsoventral markers′ expressions. For the dorsal markers chd and gsc, embryos at the shield stage were dorsal views with the animal pole oriented to the top; for the ventral markers gata2 and eve1, embryos were animal-pole views at the shield stage with the dorsal side oriented toward the right. ctr, uninjected embryos as control. The ratio indicated at the right corner was the number of embryos with altered marker expression/the number of observed embryos. See also Figure S1. |
|
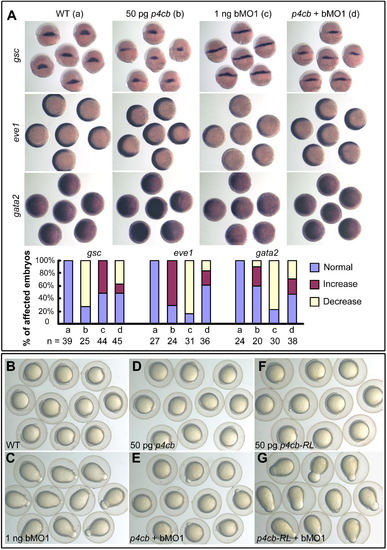
Overexpression of ppp4cb mRNA Antagonizes Dorsalizing Effect of ppp4cb Knockdown in Zebrafish Embryos (A) The rescuing effect of ppp4b (p4cb) mRNA on ppp4cb MO1 (bMO1)-induced dorsalized phenotype at the shield stage, as demonstrated by the expression of the dorsal marker gsc and the ventral markers eve1 and gata2. The statistical data are shown in the bar graphs with the number of observed embryos indicated below. (B–G) ppp4cb MO1 (bMO1)-induced dorsalized phenotype (elongated shape) at the bud stage was rescued by coinjection of ppp4cb mRNA, but not ppp4cb-RL (p4cb-RL) mRNA. See also Figure S2. |
|
Genetic Interactions between Ppp4c and Components of the BMP Signaling Pathway (A and B) Marker alterations at the shield stage caused by 10 pg bmp2b mRNA or 1 ng ppp4cb-MO1 (bMO1) were mutually inhibited by their coinjection. gsc, dorsal views; gata2 and eve1, animal-pole views with dorsal to the right; foxi1, lateral views with dorsal to the right. Statistical data for marker expression are shown in (B). Blue, red, and yellow indicated normal, increased, and decreased expression levels, respectively. The number of observed embryos was indicated. (C–H) ppp4cb-MO1 (bMO1, 1 ng)-induced elongated shape at the bud stage was rescued by coinjection of bmp2b (10 pg) or casmad5 mRNA (100 pg) encoding constitutively active Smad5. See also Figure S3. |
|
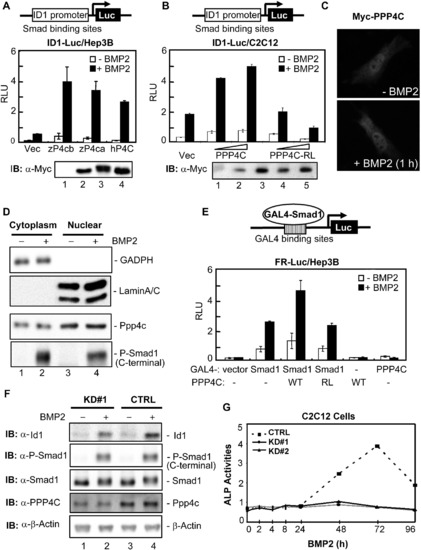
PPP4C Enhances BMP Signaling in Mammalian Cells (A) Zebrafish and human PPP4C enhanced the BMP2-induced ID1-Luc reporter expression. Indicated plasmids were cotransfected with ID1-Luc into Hep3B cells. The luciferase activity was measured 20 hr after BMP2 stimulation. The expression levels of transfected plasmids are shown in order in the lower panel. IB, immunoblot; RLU, relative luciferase activity; Vec, control vector pRK5; zP4ca and zP4cb, zebrafish Ppp4ca and Ppp4cb; hP4C, human PPP4C. (B) Human PPP4C required its phosphatase activity to enhance ID1-Luc reporter expression. PPP4C-RL is a phosphatase-dead mutant of human PPP4C. The expression levels of transfected plasmids are shown in order in the lower panel. (C) Overexpressed Myc-PPP4C in C2C12 cells was visualized by indirect immunofluorescence with or without BMP2 stimulation for 1 hr. (D) Endogenous Ppp4c was detected by western blot in both cytoplasmic and nuclear fractions of C2C12 cells. GADPH and LaminA/C served as the cytoplasmic and nuclear protein loading controls, respectively. C-terminal phosphorylation of Smad1 was detected with an anti-Phospho-Smad1 antibody (Cell Signaling Technology). (E) PPP4C enhanced the transcriptional activity from a Smad1-occupied promoter. FR-Luc reporter was transfected into Hep3B cells together with the combinations of GAL4-Smad1 (GAL4-S1) and Myc-tagged PPP4C (WT) or mutant PPP4C-RL (RL). The luciferase activity was measured after cells were treated with or without BMP2 for 20 hr. (F) Ppp4c depletion in C2C12 cells inhibited BMP-induced expression of endogenous Id1. Cell lysates were prepared from Ppp4c-KD#1 and control (CTRL) C2C12 cells stimulated with BMP2 for 2 hr. The expression levels of various proteins were examined by western blot. β-Actin served as the loading control. (G) Ppp4c depletion in C2C12 cells inhibited BMP2-induced osteoblastic differentiation. C2C12 cells were treated with BMP2 for up to 4 days to induce osteoblastic marker expression. Cell lysates were harvested at indicated time points and subjected to analysis of ALP activity. The time course of ALP activity was plotted. Data are presented with mean ± SD. See also Figure S4. |
|
Ppp4c Is a Direct Interacting Partner of Smad1 and Smad5 (A–C) Ppp4c interacted with Smad1 and Smad5 at endogenous level in C2C12 cells. The indicated proteins were immunoprecipitated or detected using corresponding antibodies. WCL, whole-cell lysate; IP, immunoprecipitation; IB, immunoblot. (D) PPP4C directly interacted with MH1 and MH2 domain of Smad1. In-vitro-synthesized full-length PPP4C and control GFPs were S35 labeled and incubated with GST protein fused with WT Smad1 (GST-Smad1) or mutant forms. Retrieved proteins were detected by autoradiography (top); Coomassie blue staining demonstrated the amount of used GST proteins (bottom). (E and F) Smad1 directly interacted with PPP4C. In vitro GST-pull-down assays were similarly conducted as in (D). The results are summarized in a schematic view in (F). (G) A schematic view of PPP4C-Smad1 interactions. (H) PPP4C deletion mutants competed with PPP4C for binding to Smad1. CoIP analysis of Smad1-PPP4C interaction in the presence of coexpressed PPP4C truncation mutants was conducted in HEK293T cells. (I and J) PPP4C truncation mutants inhibited BMP2-induced ID1 reporter activity (I) and FR-Luc reporter activity (J). (K) Endogenous Ppp4c in zebrafish embryos bound to the promoter of ventral genes eve1, gata2, and foxi1. Zebrafish embryos at the shield stage were subjected to ChIP assays using anti-PPP4C antibody, followed by detection of endogenous promoter DNA by PCR. Anti-Rabbit-IgG ChIP served as negative control. (L) Effective depletion of Smad1/Smad8 in C2C12 stables. Whole-cell lysates from C2C12 cells stably expressing pSRG vector (CTRL) or shRNA-Smad1/Smad8 (S1/8-KD) were analyzed by western blot with indicated antibodies with β-actin blot as loading control. (M) BMP2 induced Ppp4c binding to the endogenous Id1 promoter. On the top is the diagram showing the PCR-amplified region on endogenous Id1 promoter. On the bottom is the ChIP result. Depletion of Smad1/Smad8 inhibited BMP2-induced Ppp4c binding to the Id1 promoter in C2C12 cells. Anti-Rabbit IgG ChIP served as negative control. Data are presented with mean ± SD. |
|
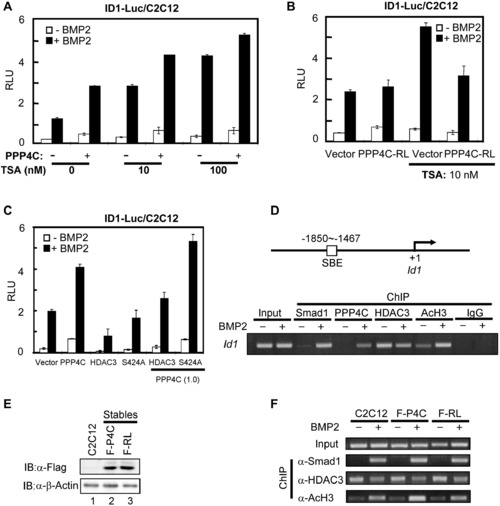
PPP4C Potentiating BMP Signaling Requires Its Phosphatase Activity to Antagonize HDAC3 Activity (A) PPP4C and HDAC inhibitor TSA similarly enhanced BMP-induced ID1-Luc reporter activity. Plasmids encoding PPP4C and ID1-Luc reporter were transfected into C2C12 cells. The luciferase activity was measured after cells were treated with or without BMP2 for 20 hr in the presence of vehicle or TSA at 10 or 100 nM. (B) Upregulation of BMP signaling by TSA treatment was blocked by PPP4C-RL (PPP4C-dead mutant). C2C12 cells transfected with plasmids encoding PPP4C-RL and ID1-Luc reporter were subjected to luciferase assays. (C) PPP4C enhanced BMP-induced ID1 promoter activity through antagonizing HDAC3 activity. C2C12 cells transfected with a combination of indicated plasmids were subjected to luciferase assays. In the parentheses is the amount of indicated plasmids (μg/4 well). S424A, an HDAC3 mutant that is refractory to PPP4C activity. (D) ChIP analysis of Id1 promoter occupancy by Smad1, Ppp4c, Hdac3, and AcH3 in response to BMP2 stimulation. C2C12 cells were treated with or without BMP2 for 2 hr; cell lysates were subjected to ChIP assays using indicated antibodies. Anti-Rabbit IgG ChIP served as negative control. Bottom view shows that Id1 promoters were detected by gel electrophoresis after regular PCR; top view is a diagram showing the PCR-amplified region on the Id1 promoter. (E) Comparable ectopic expression level of PPP4C and PPP4C-RL in C2C12 stables. Whole-cell lysates from C2C12 cells stably expressing Flag-PPP4C (F-P4C) or Flag-PPP4C-RL (F-RL) as well as the parental C2C12 cells were analyzed by western blot with indicated antibodies. β-Actin blot served as loading control. (F) PPP4C enhanced histone acetylation at the Id1 promoter in phosphatase activity-dependent manner. ChIP analysis in C2C12 stables and parental C2C12 cells was similarly conducted as in (D). Data are presented with mean ± SD. See also Figure S5. |
|
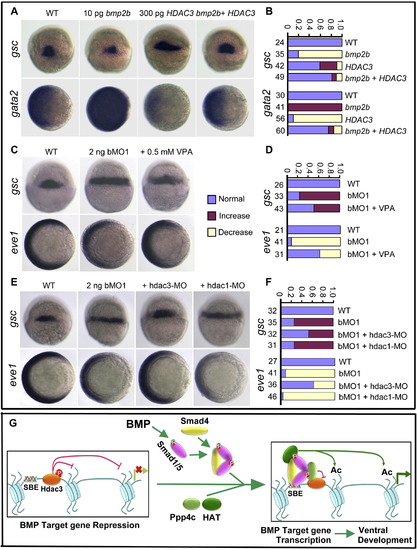
Downregulation of Hdac3 Activity Counteracts ppp4cb-MO1-Induced Embryonic Dorsalization (A and B) bmp2b mRNA-induced gsc reduction (dorsal views) and gata2 expansion (animal-pole views) were rescued by coinjection of HDAC3 mRNA. Statistical data are shown in (B). (C and D) ppp4cb-MO1 (bMO1)-induced gsc expansion (dorsal views) or eve1 reduction (animal-pole views) was inhibited by coinjection of VPA. Statistical data are shown in (D). (E and F) ppp4cb-MO1 (bMO1)-induced gsc expansion (dorsal views) or eve1 reduction (animal-pole views) was blocked by coinjection of 10 ng hdac3-MO, but not by coinjection of 10 ng hdac1-MO. Statistical data are shown in (F). (G) Schematic diagram summarizes the working model of Ppp4c in regulating BMP signaling. In response to BMP, Ppp4c is recruited to the Smad1/Smad5-occupied promoter and dephosphorylates/inactivates Hdac3. The HAT complex most probably associates with the heteromeric Smads complex, catalyzes the histone tail acetylation, and ultimately favors the transcriptional activation. |
|
Effectiveness of ppp4ca/b morpholinos, related to Figure 1. In order to test the efficacy of ppp4ca-MO1, ppp4ca-MO2, ppp4cb-MO1 or ppp4cb-MO2, the p4ca-5′UTR-GFP and p4cb-5′UTR-GFP constructs were generated. The 5′UTR containing targeting sequences of MOs and a portion of the coding sequence of ppp4ca or ppp4cb were amplified and inserted in frame to pEGFP-N3 (Clontech) with HindIII and KpnI sites. The plasmid DNA was injected alone or co-injected with each morpholino into one-cell embryos. The injected embryos were observed for GFP expression at the late gastrulation stage. Note that GFP expression was inhibited by specific morpholinos (C, D, G, H) but not by control morpholinos (B, F). |
|
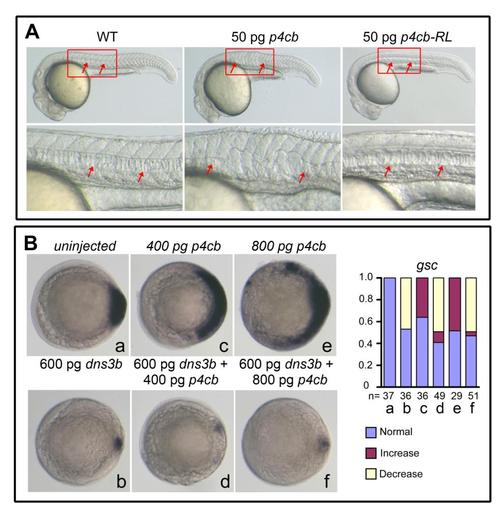
Effects of ppp4cb or its mutant overexpression, related to Figure 2. (A) 50 pg of ppp4cb mRNA (p4cb) or ppp4cb-RL mRNA (p4cb-RL) encoding the phosphatase-dead mutant form of Ppp4cb was injected at the one-cell stage and injected embryos were observed at 24 hpf. Boxed areas were enlarged to see the notochord that was indicated by arrows. p4cb overexpression interrupted the notochord development, but p4cb-RL overexpression did not cause any detectable morphological defects, which suggests that the phosphatase activity of Ppp4c is required for Ppp4c function in zebrafish embryos. (B) The embryos were injected at the one-cell stage with indicated mRNAs and examined for gsc expression at the shield stage. The statistical data were shown in the right. Notably, higher doses of ppp4cb mRNA resulted in an expansion of gsc, which was inhibited by co-injection of dnsmad3b mRNA encoding the dominant negative form of Smad2/3 (Jia et al. 2008), suggesting that too much Ppp4cb enhances Smad2/3 activity. |
|
Ppp4cb knockdown or overexpression has little effect on Wnt target gene expression, related to Figure 3. One-cell stage embryos were injected with 1 ng ppp4cb-MO1 (bMO1) or 100 pg ppp4cb mRNA and examined by whole-mount in situ hybridization for the expression of boz at the sphere stage and vent at the shield stage. boz is a direct target of maternal Wnt signaling and vent is a target of zygotic Wnt signaling. Note that both of them were not markedly affected in down- or up-regulation of ppp4cb. Upper panel, lateral views with dorsal to the right; lower panel, animal-pole views with dorsal to the right. The ratio indicated at the right corner was the number of embryos with the phenotype shown/the number of observed embryos. |
|
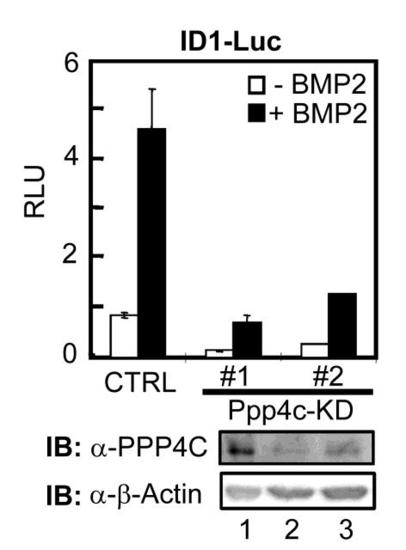
Depletion of Ppp4c inhibits BMP2-induced expression of ID1-Luc reporter in C2C12 cells, related to Figure 4. Two independent C2C12 stable clones, Ppp4c-KD #1 and #2, were selected following transfection with Ppp4c shRNA-14. The base level and BMP2-induced ID1-Luc reporter activity in these cells was dramatically decreased in cells depleted of Ppp4c. CTRL, pSRG vector. Ppp4c knockdown effect was demonstrated by Western blot in lower panel. |
|
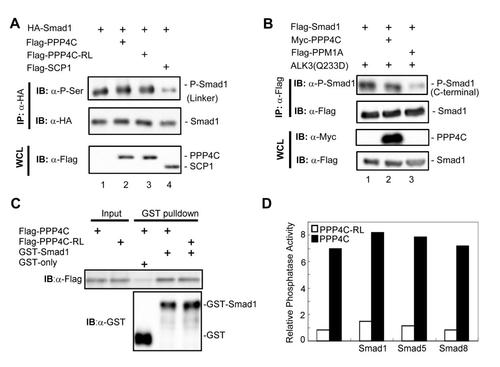
Relationship between PPP4C phosphatase activity and Smad1/5/8 phosphorylation, related to Figure 6. (A, B) To test whether PPP4C regulates Smad1 phosphorylation, we examined Smad1 phosphorylation in its linker region (A) and in its C-terminal SXS motif (B) using corresponding antibodies. Whole cell lysates (WCL) were prepared from HEK293T cells transfected with plasmids as indicated. C-terminal phosphorylation was induced by co-expressed BMP Type I receptor constitutively active mutant ALK3(Q233D). SCP1 is a known Smad1 linker region phosphatase; PPM1A is a Smad1 C-terminal phosphatase. (C) The binding of PPP4C or PPP4C-RL mutant to Smad1 was examined by GST-pulldown assay conducted as in Figure 5D. Flag-tagged PPP4C or Flag-PPP4C-RL was transfected into 293T cells. Cell lysates were harvested and subjected to the GST pull-down analysis by using recombinant GST-Smad1. GST-Smad1 retrieved PPP4C or RL mutants were examined by Western blot analysis with anti-Flag antibody. (D) In order to test whether Smads affect PPP4C phosphatase activity, we examined the ability of PPP4C to dephosphorylate pNPP substrate (See Experimental Procedures) in the presence of BMP-type specific R-Smads including Smad1, Smad5 or Smad8. PPP4C or Smad proteins (Flag-tagged) were transiently expressed in HEK293T cells and then immunoprecipitated using anti-Flag antibodies. Protein expression was determined by Western blot analysis (data not shown) to ensure comparable amount of protein used in the assays. PPP4C phosphatase activity assays were conducted as described in Experimental Procedures. The relative phosphatase activity was calculated by the absorption of p-nitrophenol at 405 nm subtracting the blank value. The data shown here were representative of experiments that were conducted two independent times. |
Reprinted from Developmental Cell, 22(5), Jia, S., Dai, F., Wu, D., Lin, X., Xing, C., Xue, Y., Wang, Y., Xiao, M., Wu, W., Feng, X.H., and Meng, A., Protein Phosphatase 4 Cooperates with Smads to Promote BMP Signaling in Dorsoventral Patterning of Zebrafish Embryos, 1065-1078, Copyright (2012) with permission from Elsevier. Full text @ Dev. Cell