- Title
-
Kupffer's vesicle is a ciliated organ of asymmetry in the zebrafish embryo that initiates left-right development of the brain, heart and gut
- Authors
- Essner, J.J., Amack, J.D., Nyholm, M.K., Harris, E.B., and Yost, H.J.
- Source
- Full text @ Development
|
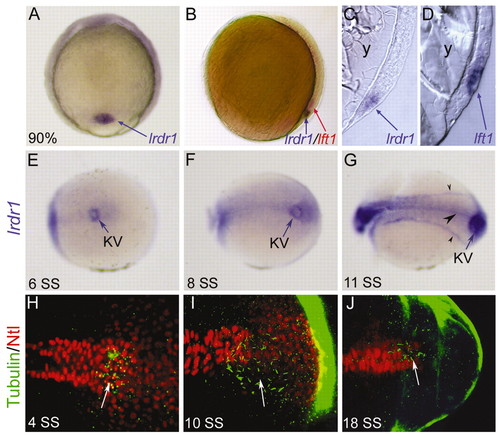
lrdr1 is expressed in dorsal forerunner cells (DFCs) and in ciliated Kupffer′s vesicle (KV). (A) lrdr1 is expressed exclusively in DFCs (arrow) at 90% epiboly. Dorsal view, anterior towards the top. (B) Lateral view of an embryo (dorsal on the right) at 80% epiboly stained for lrdr1 (purple) and lft1 (red) expression. lrdr1 is restricted to DFCs, whereas lft1 is expressed in margin cells and midline cells but not DFCs. Sectioned embryos confirmed that lrdr1 (C) is expressed in only DFCs and that lft1 (D) is expressed in cells adjacent to DFCs at 90% epiboly. No expression of lrdr1 or lft1 was detected in the yolk (y). (E-G) lrdr1 RNA expression in KV (arrow) at (E) 6 SS, (F) 8 SS and (G) 11 SS. lrdr1 was also detected in the tailbud at 8 SS (F) and in the floor plate, notochord and intermediate mesoderm (arrowheads) by 11 SS (G). Posterior-dorsal views with the anterior to the left. (H-J) Immunofluorescence at (H) 4 SS, (I) 10 SS and (J) 18 SS using anti-acetylated Tubulin antibodies (green) to detect cilia and anti-ntl antibodies (red) to label nuclei in the notochord and KV. Arrows indicate cilia in KV. Embryos were transversely bisected for visualization of the tailbud. EXPRESSION / LABELING:
|
|
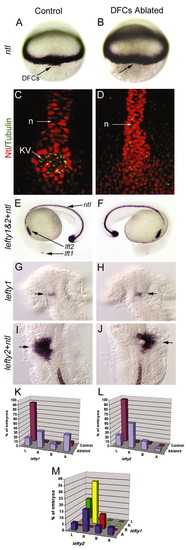
Laser ablation of DFCs alters LR patterning without affecting development of the midline. Unablated control embryos expressed ntl in DFCs (arrow) at 60% epiboly (A) and in the notochord at 24 hours post-fertilization (E). Laser ablation eliminated DFCs (arrow, dorsal view at 60% epiboly) but did not alter ntl expression in equatorial mesoderm (B) or in notochord and tailbud at 24 hpf (F). Control embryos (C) and DFC-ablated embryos (D) were immunostained with anti-acetylated Tubulin antibodies (green) to detect cilia and anti-ntl antibodies (red) to detect the notochord (n) and Kupffers vesicle (KV). Both control and DFC-ablated embryos showed contiguous ntl staining in the notochord, even in embryos (n=2/6) that failed to form KV (D). (G-J) In control embryos, we observed normal expression of lft1 (arrow) in the left dorsal diencephelon (G) and lft2 (arrow) in the left heart primordia (I). In DFC-ablated embryos, lft1 (arrow) in the diencephalon (H) and lft2 (arrow) in the heart primordia (J) were frequently reversed. (K-M) Analysis of lft1 expression in the diencephalon (K) and lft2 expression in the heart primordia (L) in control (n=71) and DFC-ablated (n=17) embryos. (M) Analysis of lft1 expression plotted against lft2 expression in DFC-ablated embryos indicated that the predominant class of DFC-ablated embryos displayed reversal of both brain and heart markers (yellow bar). L, left; R, right; B, bilateral; A, absent gene expression. |
|
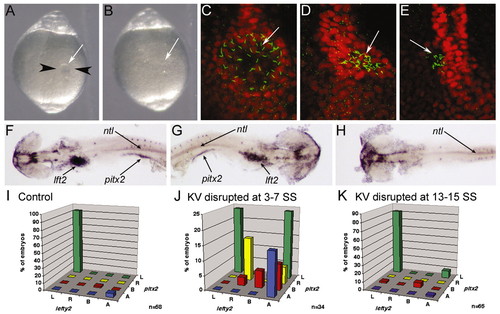
KV is essential for LR development during early somitogenesis. (A) Image of a live 6 SS embryo prior to disruption of KV. Black arrowheads represent the path of the needles during KV disruption and the white arrow indicates KV. (B) Image of the same embryo in A after disruption of KV. White arrow indicates where KV was previously located. (A,B) Dorsal views of the tail. (C-E) Control embryo (C) and KV disrupted embryos (D,E) stained with anti-acetylated tubulin (green) and ntl (red) antibodies at 10 SS. Arrows indicate KV cilia. (F-H) In situ hybridization analysis of lft2, pitx2 and ntl at 20-25 SS. (F) A control embryo showing normal left-lateral gene expression. KV disrupted embryos often showed either reversed (G) or an absence (H) of lateral gene expression. Arrows indicate lft2 staining in the heart primordia, pitx2 staining in the lateral mesoderm and ntl staining in the notochord. Yolk was dissected away for visualization of lateral staining. (F-H) Dorsal views with the anterior towards the left in F,H and to the right in G. (I-K) lft2 expression in the heart primordia plotted against pitx2 expression in the lateral plate mesoderm in control (I), KV-disrupted at 3-7 SS (J) and KV-disrupted at 13-15 SS (K) embryos. L, left; R, right; B, bilateral; A, absent. n, number of embryos examined. EXPRESSION / LABELING:
|
|
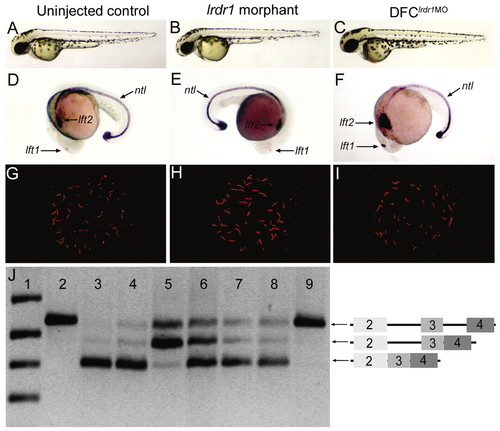
Morpholino knockdown of lrdr1 in all embryonic cells or specifically in DFCs perturbs LR patterning without affecting midline or KV development. (A-C) The majority of embryos injected with either 1 ng lrdr1-AUG MO (B) or 1.6 ng lrdr1E2/I2 MO (not shown) at the one- to four-cell stages (lrdr1 morphants) and embryos in which 1 ng lrdr1-AUG MO + 1.6 ng lrdr1E2/I2 MO was co-injected into the yolk at mid-blastula stages to target DFCs (DFClrdr1MO embryos) (C) appeared similar to uninjected controls (A) at 2 dpf. (D-F) ntl expression in the notochord was normal in lrdr1 morphants (E) and DFClrdr1MO embryos (F), but expression of the normally left-sided markers lft1 and lft2 (D) was altered, including right-sided (E) and bilateral (F) expression. (G-H) KV cilia were detected by anti-Tubulin antibodies (red) in both lrdr1 morphants (H) and DFClrdr1MO embryos (I), and resembled cilia in wild-type controls (G). (J) RT-PCR analysis of RNA extracted from embryos between 80-90% epiboly was used to determine the efficacy of lrdr1E2/I2 MO in DFCs. Primers in lrdr1 exons 2 and 4 amplified a fragment of the gene that includes exons 2-4 and introns 2-3 from genomic DNA (lane 2). The predominant cDNA fragment amplified in uninjected embryos (lane 3) and embryos injected with 1 ng of control MO at the one- to four-cell stages (lane 4) corresponds to spliced lrdr1 mRNA (exons 2-4). lrdr1E2/I2 MO (1.6 ng) injected at the one- to four-cell stages (lane 5) or at mid-blastula stage (lane 6) resulted in the accumulation of lrdr1 transcripts that retain intron 2. This was not observed in embryos injected with lrdr1E2/I2 MO between dome stage and 30% epiboly (lane 7) or with 1.5 ng lft1 MO at mid-blastula stages (lane 8). Negative control reactions that lacked reverse transcriptase (lane 9) amplified only a genomic fragment that probably reflects genomic DNA contamination in RNA extracts. DNA ladder was loaded in lane 1. Diagrams on the right represent the amplicons confirmed by direct sequencing of RT-PCR products. EXPRESSION / LABELING:
|
|
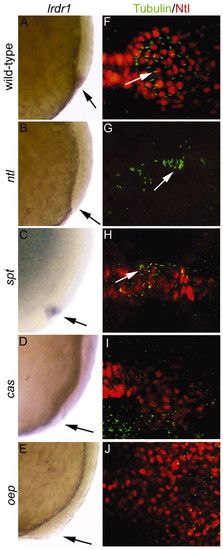
ntl and the Nodal signaling pathway regulate lrdr1 expression, and, with other genes, control morphogenesis of Kupffer′s vesicle. Wild-type (A,F) and mutant ntlb195(B,G), sptb104(C,H), casta56 (D,I) and oepm134 (E,J) embryos were examined for lrdr1 mRNA expression, cilia formation and KV organization. (A-E) lrdr1 expression at 80-90% epiboly. Lateral views, dorsal towards the right, anterior upwards; arrows indicate DFCs. (F-J) Immunofluorescence analysis of cilia and KV morphogenesis using anti-acetylated Tubulin antibodies (cilia, green) and anti-Ntl antibodies (KV, red) at 6-10 SS. Dorsal views with anterior towards the left. Arrows indicate cilia. EXPRESSION / LABELING:
|
|
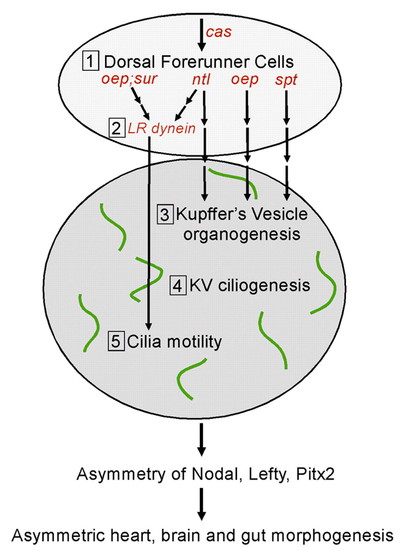
Multiple genetic pathways control the formation and function of Kupffer′s vesicle to direct LR development. We propose five steps (1-5) that are required to set up normal asymmetric expression patterns of Nodal, Lefty and Pitx2 gene family members and subsequent morphological asymmetry of the heart, brain and gut in the zebrafish embryo. First, DFCs are specified during the blastula period. Probably among a number of yet unidentified factors, cas is required for proper DFC formation. Second, the ntl and oep (Nodal signaling) pathways control the expression of Left-Right dynein (lrdr1), a ciliary motor protein, in DFCs. Third, DFCs organize to form KV during early somitogenesis. ntl and oep are also essential for this step. By contrast, spt is required for KV organogenesis but not for lrdr1 expression in DFCs. Fourth, cilia assemble on cells lining the fluid-filled lumen of KV. Fifth, KV cilia become motile, which is dependent on left-right dynein, and generate a directional flow of fluid inside KV. We suggest that fluid flow inside KV, a transient embryonic organ of asymmetry, initiates asymmetric gene expression in lateral tissue that leads to normal LR development. |
|
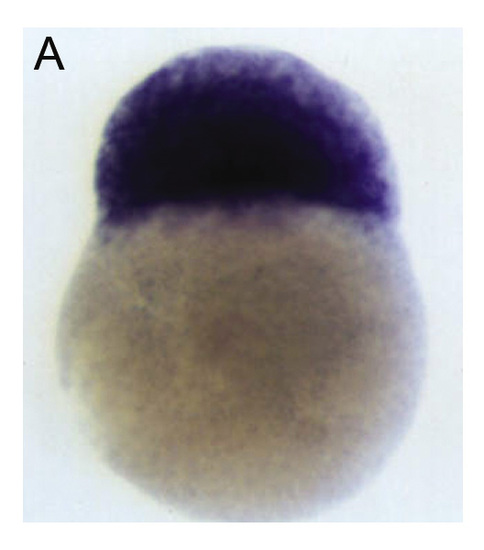
lrdr1 is supplied maternally. (A) In situ shows ubiquitous maternal lrdr1 mRNA in embryonic cells at the 256-cell stage prior to the activation of zygotic genes. EXPRESSION / LABELING:
|

Unillustrated author statements EXPRESSION / LABELING:
|