Fig. 4
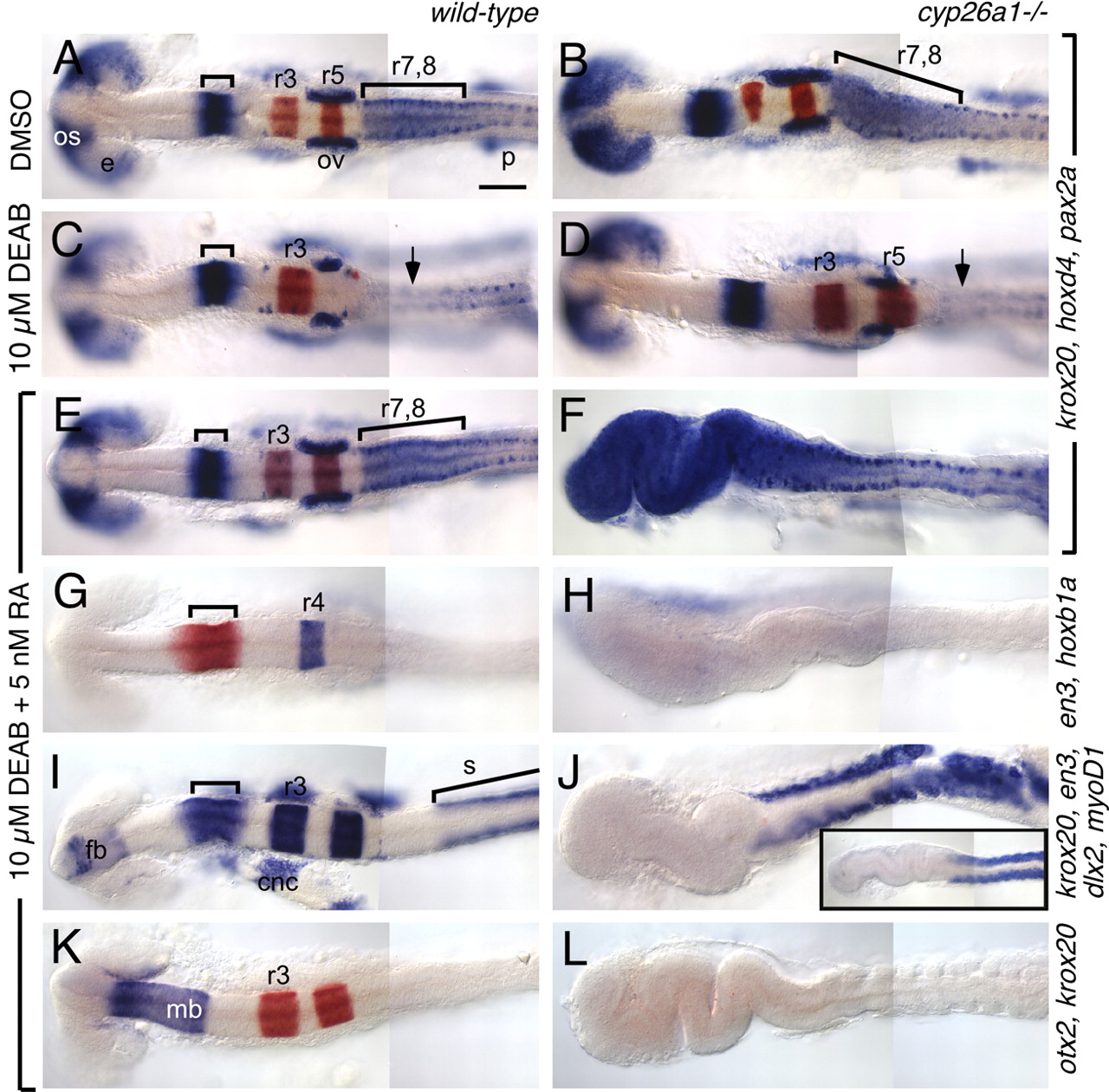
Fig. 4 cyp26a1 protects the hindbrain from exogenous RA. Wild-type (left column) and cyp26a1-/- (right column) embryos treated with DMSO (A,B), 10 μM DEAB (C,D) or 10 μM DEAB + 5 nM RA (E-L). RNA in situ hybridizations use the markers described in Fig. 2, except for I,J, which is a mix of en3 (bracket), krox20 (r3, r5), dlx2 [cranial neural crest (cnc) and forebrain (fb)] and myoD (somites; s). Large bracket in A indicates the r7-r8 region, which is elongated in cyp26a1 mutants (B). (C) In DEAB-treated embryos, posterior rhombomeres (r5-r8) are absent (arrow indicates the absence of high hoxd4 expression characteristic of r7-r8). (D) This phenotype is partially rescued in cyp26a1 mutants, as seen by rescue of r5 but not of r7-r8. (E-L) The DEAB phenotype is fully rescued in wild-type embryos by treatment with 5 nM RA (E,G,I,K) whereas, in cyp26a1 mutants, this low dose of RA causes strong posteriorization of the brain (F,H,J,L). This phenotype resembles that of wild-type embryos treated with 200 nM RA (inset in J). Scale bar: 100 μm. os: optic stalk; e: eye; p: pronephros.