- Title
-
Identification of renal stem cells in zebrafish
- Authors
- Yu, T., Liu, X., Tan, X., Zhang, Y., He, Z., Yang, W., Tian, T., Li, Y., Zhao, J., Liu, C.
- Source
- Full text @ Sci Adv
|
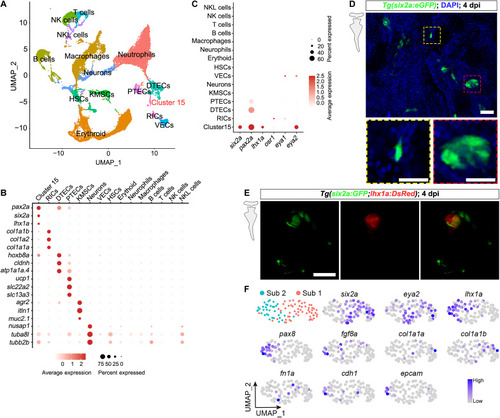
Identification of potential zebrafish RSC clusters via scRNA-seq analysis of injured kidneys. ( |
|
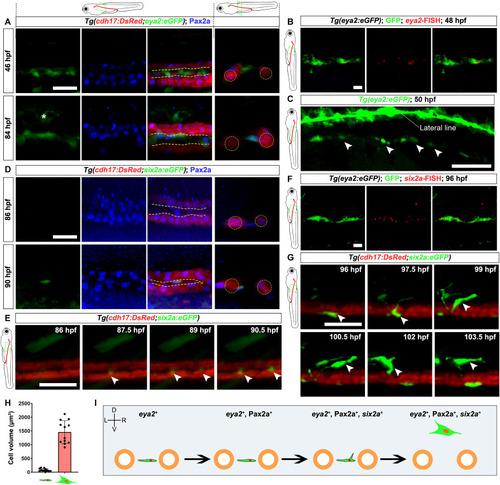
RSC generation process. ( |
|
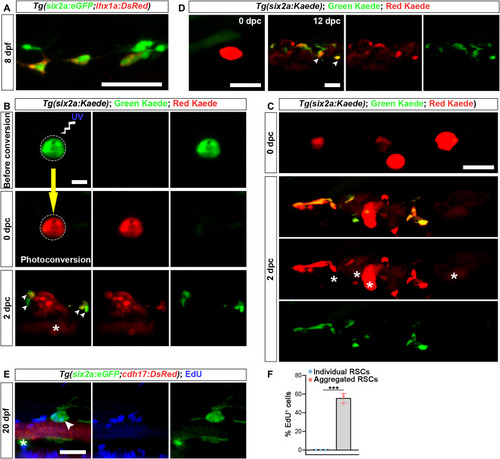
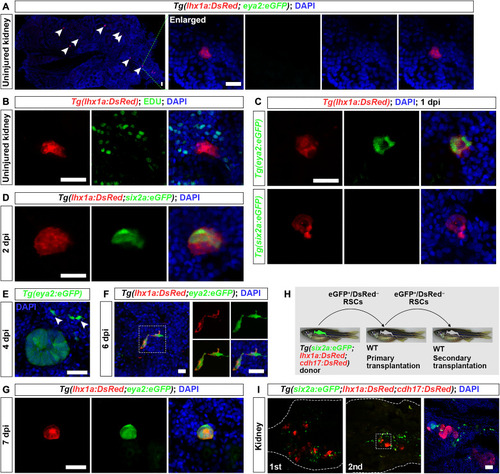
Renewal of RSCs. ( |
|
MET and EMT processes during RSC renewal. ( |
|
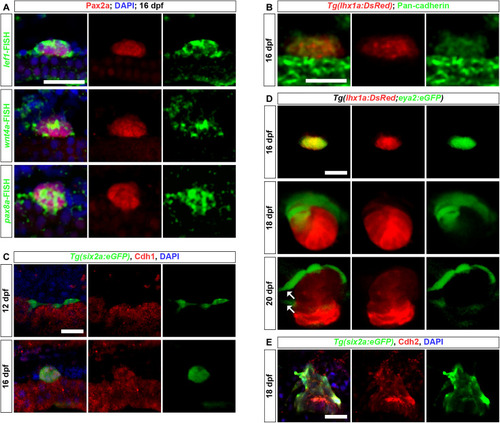
Renewal of RSCs in adult kidneys. ( |
|
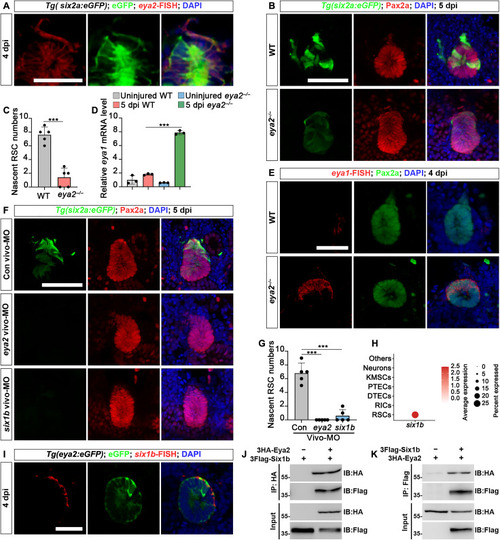
Eya2-Six1b complex regulates RSC renewal. ( |
|
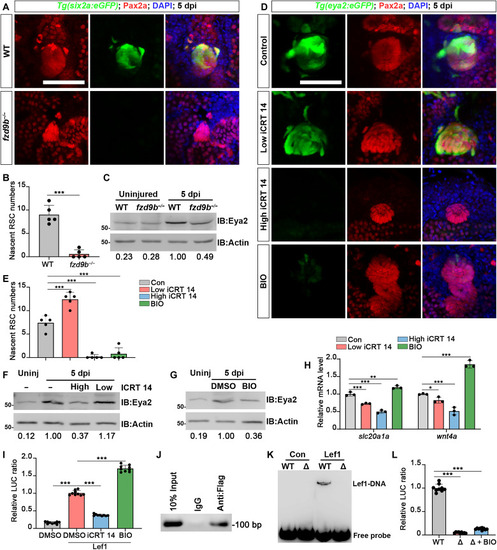
Wnt regulates the renewal of RSCs by directly activating ( |
|
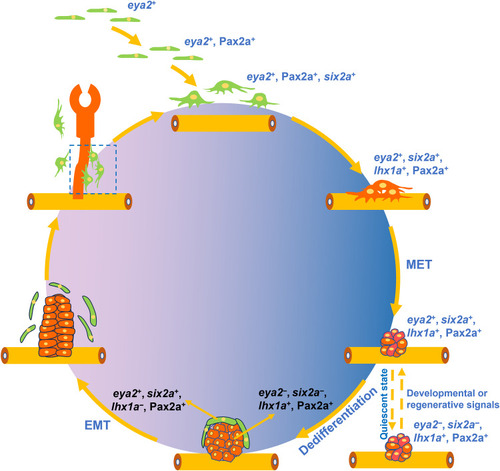
Schematic overview of zebrafish RSC renewal and differentiation. During zebrafish embryonic development, RSCs arise from |