- Title
-
Spatiotemporal transition in the role of synaptic inhibition to the tail beat rhythm of developing larval zebrafish
- Authors
- Roussel, Y., Paradis, M., Gaudreau, S.F., Lindsey, B.W., Bui, T.V.
- Source
- Full text @ eNeuro
|
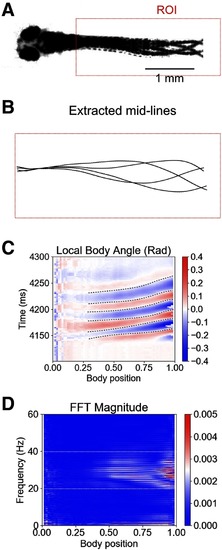
Larval zebrafish tail beat frequency during swimming is mainly between 20 and 40 Hz. |
|
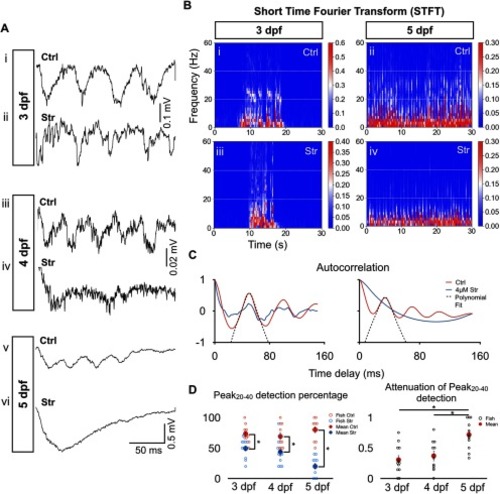
Greater effect of strychnine on tail beat rhythmicity at 5 dpf than at 3–4 dpf. |
|
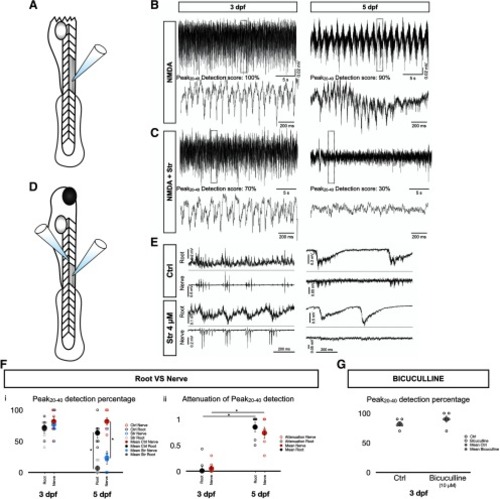
Transition to a SGDR driving tail beats observed in various conditions. |
|
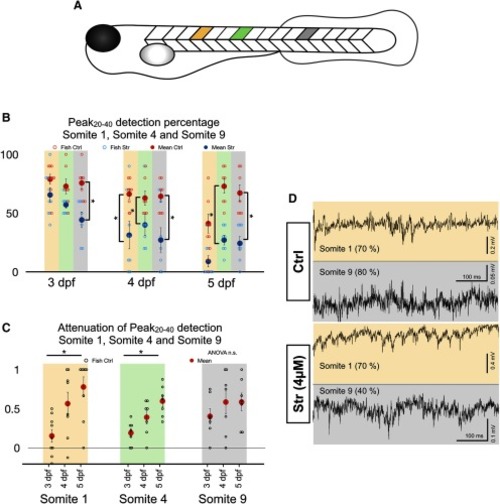
Differential effect of strychnine along the rostro-caudal axis of the zebrafish. |
|
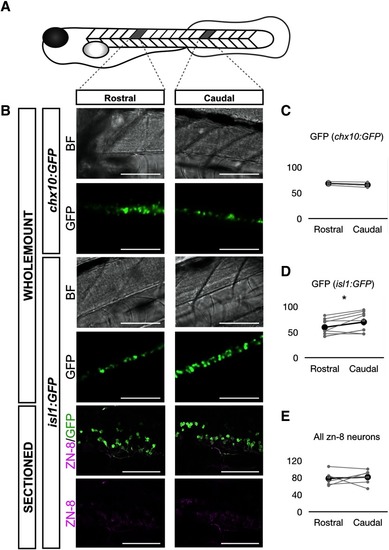
Cell count of sMNs and chx10+ spinal neurons in 3-dpf larval zebrafish. |
|
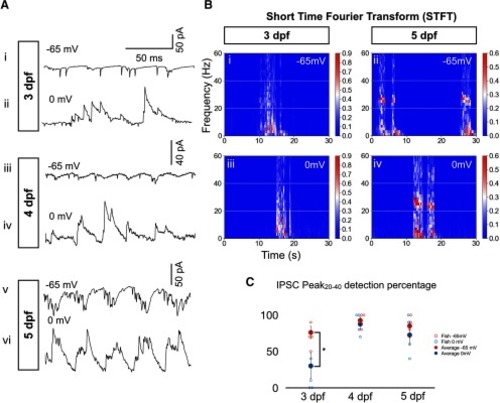
IPSCs mature from arrhythmic at 3 dpf to rhythmic with a frequency close to that of tail beats at 5 dpf during swimming episodes. |
|
SGDR is preserved in MTZ-treated Tg( |
|
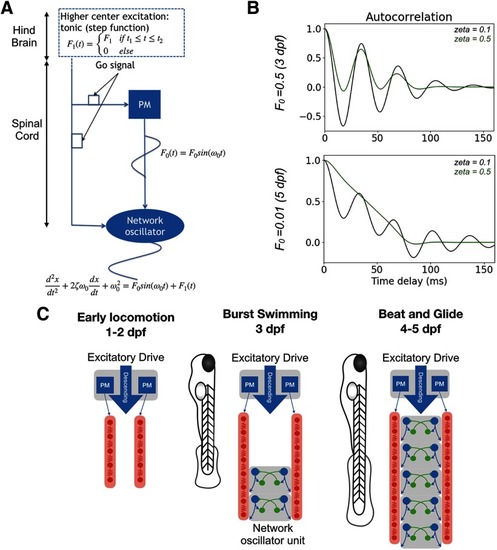
Coupled oscillator model of architectural change from a pacemaker to a network oscillator-based spinal locomotor circuits of developing zebrafish. |