- Title
-
Regulation of posterior body and epidermal morphogenesis in Zebrafish by localized Yap1 and Wwtr1
- Authors
- Kimelman, D., Smith, N.L., Lai, J.K.H., Stainier, D.Y.
- Source
- Full text @ Elife
|
yap1;wwtr1 double mutants exhibit severely altered posterior development. (A) Schematic showing the two mutants used in this work. TEAD BD is the TEAD binding domain, TAD is the Transcriptional Activation Domain, and WW, SH and PDZ are protein interaction domains. The black bar indicates new sequence following the frame shift caused by the mutation. (B) Time course showing the development of the posterior body defect in yap1;wwtr1 double mutants. The same sibling and double mutant embryos are photographed at all three stages. (C) Phalloidin staining shows that the somites form in the mutants but never acquire the chevron shape found in wild-type embryos. 24-somite stage (21 hpf) embryos, anterior to the left. (D) Embryos with a yap1-/-;wwtr1+/- genotype show a milder posterior body defect at 72 hpf (n = 15). Pericardial edema is also variably present. (E,F) Midline confocal sections of the trunk with somite 1 on the left side of a sibling (E) and a yap1;wwtr1 double mutant embryo (F) at the 24-somite stage. PHENOTYPE:
|
|
Yap1 is localized to the presumptive epidermis and axis. (A) Yap1 protein is expressed in the presumptive epidermis (arrows) and midline (arrowhead) of an 18-somite stage embryo. (B) Co-stain with the notochord marker no tail (ntl), in green, using FISH demonstrates Yap1 expression, in red, in the notochord. (C) No reactivity with the Yap1 antibody was observed in yap1-/- mutant embryos. Note that the longer Proteinase K digestion used in the standard FISH protocol reduced the presumptive epidermal Yap1 staining. All embryos are at 18 somites with anterior to the left. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Yap1 localizes to the presumptive epidermis. A combination of FISH and immunofluorescence was used to co-stain embryos for Yap1 protein and keratin 4 or keratin 8 mRNA, which are restricted to the presumptive epidermis. A brief (5 min) Proteinase K step was used for the FISH, which preserves the presumptive epidermal Yap1 stain but does not allow for strong notochord staining. |
|
Wwtr1 and medaka Yap1 localize to the presumptive epidermis and notochord. (A–C) Wwtr1 expression in zebrafish embryos. Wwtr1 is expressed in the presumptive epidermis (arrow) and notochord (arrowhead) of an 18-somite embryo (A). Co-stain with the notochord marker no tail (ntl) (in green) demonstrates Wwtr1 expression (in red) in the notochord (B). No reactivity with the anti-Wwtr1 antibody was observed in wwtr1 mutant embryos (C). All embryos are 18 somites with anterior to the left. (D) Yap1 is expressed in the presumptive epidermis (arrow) and notochord (arrowhead) of an 18-somite medaka embryo. EXPRESSION / LABELING:
PHENOTYPE:
|
|
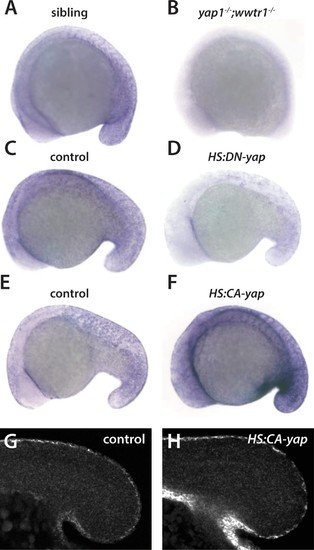
Regulation of ecrg4b expression by Yap1 and Wwtr1. (A,B) ecrg4b is expressed in the presumptive epidermis of sibling embryos (A) but is not expressed in yap1;wwtr1 double mutant embryos (B). (C–F) Transgenic embryos obtained from an outcross of hemizygous HS:DN-yap or HS:CA-yap adults were heat shocked at the 4-somite stage and then raised to 18 somites. The genotype of the embryos was determined by PCR after the in situ hybridization to be either transgenic or non-transgenic control. Expression of DN-yap decreased ecrg4b expression relative to non-transgenic controls (C,D, n = 6 for each) whereas CA-yap enhanced ecrg4b expression (E,F, n = 4 for each). (G,H) Embryos analyzed using FISH probe to ecrg4b demonstrates that CA-yap increases expression of ecrg4b only in the epidermis (n = 4 for each). All embryos are at the 18-somite stage. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Regulation of wu:fc23c0 by Yap1 and Wwtr1. (A,B) wu:fc23c0 is strongly expressed throughout the notochord of sibling embryos (A) but not expressed in the posterior notochord of yap1;wwtr1 double mutant embryos. (C–E) Transgenic embryos obtained from an outcross of hemizygous HS:DN-yap or HS:CA-yap adults were heat shocked at the 4-somite stage and then grown to 18 somites. The genotype of the embryos was determined by PCR after the in situ hybridization to be either transgenic or non-transgenic control. Expression of DN-yap decreased posterior notochord wu:fc23c0 expression (D, n = 10) whereas CA-yap did not appear to alter wu:fc23c0 expression (E, n = 8) relative to control non-transgenic embryos (C). EXPRESSION / LABELING:
PHENOTYPE:
|
|
Yap1 and Wwtr1 do not regulate arhgap12 and arhgap27 expression. (A,B) arhgap12a and (C,D) arhgap27 are expressed in the presumptive epidermis at the same level in sibling and yap1;wwtr1 double mutant embryos. The yolk is blue from the prolonged development times necessary to visualize these weakly expressed transcripts. EXPRESSION / LABELING:
|
|
Morphogenetic defects in noto mutants. Embryos from a cross of noton1 heterozygotes were sorted at the 16-somite stage when the noto phenotype first becomes visible. The same sibling and mutant embryos are photographed at all three stages shown. |
|
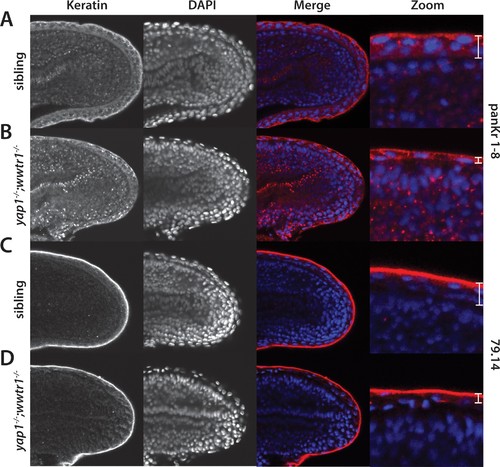
Alterations in the presumptive epidermis in yap1;wwtr1 double mutants. Mutant and sibling embryos at the 24-somite stage were fixed, incubated with the anti-keratin antibodies panKr1-8 (A,B) or 79.14 (C,D), and then co-stained with DAPI. Confocal images of the midline of the tailbud, with anterior to the left. Note the thinner presumptive epidermis in the mutants compared to that in the siblings. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Tp63 positive cells accumulate at the nascent fin fold in sibling but not mutant embryos. Embryos were incubated with the anti-Tp63 antibody, and then co-stained with DAPI. (A) Embryos were collected at the indicated stages. Note the increase in Tp63-positive cells on the dorsal and ventral midline as the embryos age. (B–D) The Tp63-positive presumptive epidermis at the 18-somite stage (B) is one layer thick, but increases to multiple layers by the 24-somite stage in sibling (C) but not in yap1;wwtr1 double mutant embryos (D). Confocal images of the midline of the tailbud, with anterior to the left. The zoomed in images in panels B-D are of the dorsal side, and the arrows in the merged images point to the rare presumptive epidermis Tp63-negative cells. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Dynamic movements of presumptive epidermal cells at the ventral fin fold. (A) Cartoon showing previous analysis of dorsal fin fold formation redrawn from Dane and Tucker, 1985. (B) Stills from live imaging of ventral fin fold formation in wild-type embryos taken from Video 2. (C) Stills from live imaging of ventral fin fold formation in yap1;wwtr1 double mutants taken from Video 3. Images are of the midline with anterior to the left. Arrowheads point to new cells arriving at the midline. Most of the cells in the wild-type arrive basally, although two cells outlined appear at the edge of the fin fold coming from the other side of the embryo. Note that in wild-type embryos most cells that are at the midline at t = 0 min, or come to the midline at later times, stay at the midline whereas in the yap1;wwtr1 double mutants cells are frequently transiently at the midline (quantified in Figure 8). Lateral views of the ventral-posterior part of the embryo, with dorsal up and ventral down. Wild-type movie is a representational movie of 21 movies examined and the mutant movie is representational of 11 movies. PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|
|
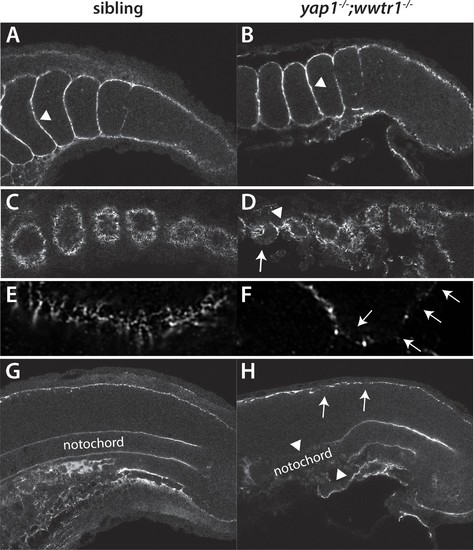
Fibronectin deposition is altered in yap1;wwtr1 double mutants. (A,B) Intersomitic Fn deposition (arrowheads) appears unaffected in sibling and mutant embryos. Lateral confocal section. (C,D) At the lateral edges of the somites, Fn is present in rosettes where the somites contact the presumptive epidermis in sibling embryos (C), whereas in yap1;wwtr1 double mutants there are gaps (arrow) and regions of enhanced Fn accumulation (arrowhead, (D). (E, F) Higher magnification views of the ventral region of a single somite near its lateral edge from sibling (E) and yap1;wwtr1 double mutant (F) embryos showing altered Fn accumulation in the mutants. Arrows point to gaps in Fn deposition. (G,H) At the midline, Fn is discontinuous underneath the presumptive epidermis in yap1;wwtr1 double mutants (arrows, (H) compared to siblings (G)). The posterior notochord Fn staining appears mostly unaffected in yap1;wwtr1 double mutants, whereas in more anterior regions the Fn staining is absent (arrowheads), unlike in the siblings. All embryos are at 18-somites with anterior to the left. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Expression of Fn in medaka embryos. (A) In a lateral section Fn protein is observed around the somites, including between adjacent somites, but does not appear within the somites as is also observed in zebrafish. (B) In a midline section, Fn is observed under the epidermis and around the notochord, as in zebrafish. Fn is also observed in periodic clusters along the notochord in medaka. |
|
Inhibition of Fibronectin activity mimics the yap1;wwtr1 double mutant phenotype. (A–C) Control embryo (A) and embryos injected with 400 pg fn∆C mRNA (B,C). (D, E) Control (D) and fn∆C-injected (E) embryos stained with phalloidin. Note the absence of chevron-shaped somites in panel E. (F, G) Control (F) and fn∆C-injected (G) embryos incubated with the anti-Tp63 antibody and co-stained with DAPI. Note the single layer of Tp63-positive presumptive epidermal cells in panel G (n = 12/12 embryos with a shortened tail had a single layered presumptive epidermis). All embryos are at the 24-somite stage with anterior to the left. |
|
Movement of cells expressing Fn∆C. (A) Stills from live imaging of ventral fin fold formation in embryos expressing Fn∆C from injected mRNA. This movie is a representational movie of one of three movies. Arrowheads show new cells entering the fin fold. (B) The time individual Fn∆C expressing cells were at the midline measured in a single movie. Compare to wild-type cells in Figure 8A. Embryos were positioned on their side and filmed from the left side. When Fn∆C cells crossed over the midline to the right side of the embryo, we stopped recording them, resulting in a truncated line. |
|
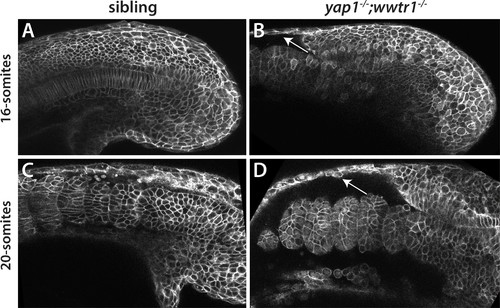
Reduced adhesion of presumptive epidermis in yap1;wwtr1 double mutants. (A–D) Embryos were injected with mRNA encoding a membrane-localized form of GFP and imaged at the indicated stages. (A,C) Sibling embryos. (B,D) yap1;wwtr1 double mutant embryos. Note the progressive separation of the epidermis from the somites (arrows), which increases from the 16-somite to the 20-somite stage. Four of four mutant embryos at the 16-somite stage exhibited the tissue separation phenotype and three of three at the 20-somite stage. PHENOTYPE:
|
|
Role of Yap1/Wwtr1 in somite stage morphogenesis. (A) Yap1 and Wwtr1 are expressed specifically in the presumptive epidermis and notochord (light blue), where they promote cell adhesion and Fn assembly (orange). These processes are essential for both the movement of presumptive epidermal cells dorsally and ventrally to form the MFF, and for force transmission of the underlying tissues to allow the elongation of the posterior body. (B) At the midline, cells are converging from either side and push against each other to form the dorsal (shown) and ventral MFFs. We propose that adhesion to Fn is required for cells to be able to create the necessary force to allow them to generate the MFF. The presomitic mesoderm is shown in red and the neural tube is green. |

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. EXPRESSION / LABELING:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. EXPRESSION / LABELING:
|