- Title
-
Tbx20 drives cardiac progenitor formation and cardiomyocyte proliferation in zebrafish
- Authors
- Lu, F., Langenbacher, A., Chen, J.N.
- Source
- Full text @ Dev. Biol.
|
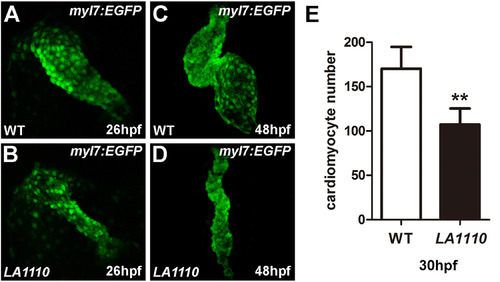
LA1110 mutants exhibit a reduction in cardiomyocyte cell number. (A and B) Tbx20 mutant embryos (B) form a thin and small heart tube at 26hpf compared with that of wild-type siblings (A), as indicated by the fluorescent labeling of differentiated cardiomyocytes by the myl7:EGFP transgene. (C and D) Wild type embryos display a looped two-chambered heart at 48hpf (C), whereas LA1110 mutant embryos (D) exhibit a thin, stretched and string-like heart. (E) Graph of the average number of GFP-positive cardiomyocytes in wild type and LA1110 mutant embryos at 30hpf (n=5). Error bars indicate standard deviations. Asterisks indicate a significant difference (**p<0.01). |
|
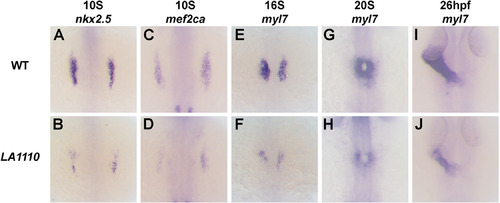
The LA1110 gene is required for cardiac progenitor formation. (A–J) Dorsal views of nkx2.5 (A and B), mef2ca (C and D) and myl7 (E–J) expression in wild type and LA1110 mutant embryos. In wild type embryos, expression of nkx2.5 (A) and mef2ca (C) are detected in the cardiac progenitors within the ALPM at the 10-somite stage. These cells then differentiate into myl7-expressing cardiomyocytes (E) by the 16-somite stage and migrate toward the midline and fuse to form a cardiac cone (G) which then grows into a linear heart tube by 26 hpf (I). In LA1110 mutant embryos, expression of nkx2.5 (B) and mef2ca (D) is strongly reduced. Even though the expression of myl7 is far lower (F), the myl7-expressing cardiomyocytes in LA1110 mutant embryos migrate to the midline and form the cardiac cone at the 20-somite stage (H) and the linear heart tube at 26 hpf (J). EXPRESSION / LABELING:
PHENOTYPE:
|
|
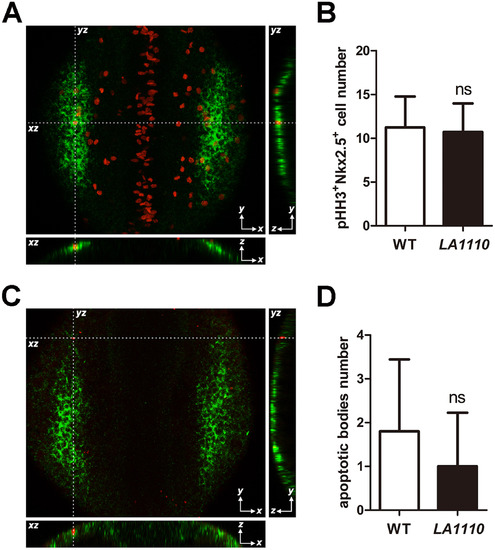
Quantification of cardiac progenitor proliferation and apoptosis in LA1110 mutants and wild type siblings. (A) Dorsal view of pHH3 immunostaining (red; nuclei) on a 10-somite stage wild type embryo. The cardiac progenitor cells are visualized by nkx2.5 fluorescent in situ hybridization (green; cytoplasmic). Orthogonal reconstructions are shown on the bottom and the right side of the image. (B) Graph of the average number of pHH3-positive cardiac progenitor cells in wild type (n=9) and LA1110 mutant embryos (n=7). (C) Dorsal view of apoptotic bodies (red) detected by TUNEL assay in a 10-somite stage wild type embryo. Cardiac progenitor cells are marked by nkx2.5 fluorescent in situ hybridization (green; cytoplasmic). Orthogonal reconstructions are shown on the bottom and the right side of the image. (D) Graph of the average number of apoptotic bodies in the cardiac progenitor region in wild type (n=5) and LA1110 mutant embryos (n=5). Error bars indicate standard deviations. ns, not significant. EXPRESSION / LABELING:
PHENOTYPE:
|
|
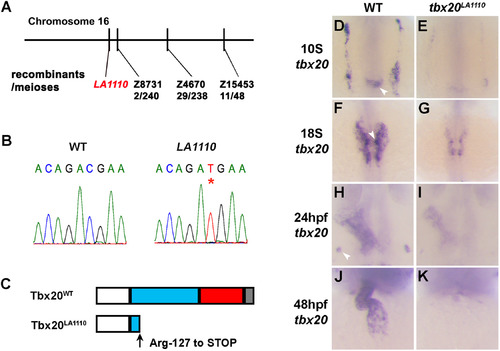
Positional cloning of the tbx20LA1110 mutant. (A) Diagram showing the location of the LA1110 locus with respect to markers on Chromosome 16. (B) Sequencing of the tbx20 locus in wild type and LA1110 mutant embryos identified a C to T transition, resulting in a premature stop codon (*). (C) Schematic diagram of the Tbx20WT and Tbx20LA1110 proteins. Zebrafish Tbx20WT contains an N-terminal domain (white), a T-box domain (blue), and predicted transactivation (red) and transrepression (grey) domains in the C-terminal portion of the protein. The Tbx20LA1110 protein is truncated within the T-box DNA binding domain. (D-K) Expression of tbx20 in wild type and LA1110 embryos showing the anterior dorsal view (D-I) and frontal view (J, K). In wild type embryos, expression of tbx20 is visible as bilateral strips in the cardiac primordia within the ALPM at the 10-somite stage (D). These strips of cells migrate toward and fuse at the midline (F). By 24 hpf, the entire heart tube displays expression of tbx20 (H, J). Additional tbx20 expression is present within the hindbrain at the 10-somite stage (arrowhead in D), hindbrain branchiomotor neurons at the 18-somite (F) and the primordia of the pituitary gland at 24 hpf (arrowhead in H). (E, G, I and K) In tbx20LA1110 mutant embryos, tbx20 transcripts are dramatically reduced. EXPRESSION / LABELING:
PHENOTYPE:
|
|
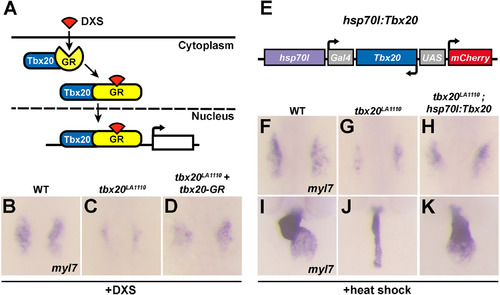
Tbx20 overexpression rescues the cardiac defects present in tbx20 mutant embryos. (A) Diagram of the inducible activity of the tbx20-GR (glucocorticoid receptor) fusion protein. The addition of DXS (dexamethasone) is required for the translocation of the tbx20-GR protein into the nucleus. (B-D) Dorsal views of myl7 expression at the 16-somite stage in wild type embryos (B), tbx20 mutants (C) and tbx20 mutants injected with tbx20-GR mRNA (D). The cardiomyocyte production defects in tbx20 mutant embryos are substantially rescued by the injection of tbx20-GR mRNA. (E) Schematic representation of the hsp70l:Tbx20 transgene used for tbx20 overexpression. (F-H) Dorsal views of myl7 expression at the 16-somite stage in wild type (F), tbx20 mutant (G) and Tbx20-/-; Tg(hsp70l:Tbx20) embryos (H). (I-K) Frontal views of myl7 expression at 48hpf in wild type (I), tbx20 mutant (J) and Tbx20-/-;Tg(hsp70l:Tbx20) embryos (K). Overexpression of Tbx20 successfully restored cardiomyocyte production in tbx20 mutant embryos. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Tbx20 overexpression significantly increases cardiomyocyte number. (A-H) Dorsal views of nkx2.5, mef2ca, scl and myl7 expression in non-transgenic (A, C, E and G) and Tg(hsp70l:Tbx20) embryos (B, D, F and H). The expression of nkx2.5 and mef2ca is expanded in Tbx20-overexpressing embryos at the 10-somite stage (A-D), whereas the expression of scl is similar to that of control embryos (E and F). Similarly, myl7 expression is enhanced in Tg(hsp70l:Tbx20) embryos at the 16-somite stage (H). (I and J) Representative confocal projections of myl7:nucGFP hearts from heat-treated non-transgenic (I) and Tg(hsp70l:Tbx20) embryos (J) at 40hpf. (K) Graph of the average number of nucGFP-positive cardiomyocytes in heat-treated non-transgenic (n=8) and Tg(hsp70l:Tbx20) embryos (n=10). Tbx20 overexpression enhanced cardiomyocyte production. (L) Graph of the ratio of BrdU-positive cardiomyocytes to the total number of cardiomyocytes in heat-treated non-transgenic and Tg(hsp70l:Tbx20) embryos (n=7). Overexpression of Tbx20 in early embryos promoted cardiomyocyte proliferation. Error bars indicate standard deviations. Asterisks indicate a significant difference (**p<0.01, ***p<0.001). EXPRESSION / LABELING:
PHENOTYPE:
|
|
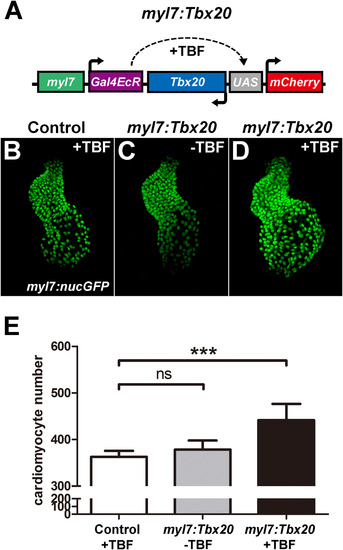
Cardiomyocyte-specific Tbx20 expression promotes cardiomyocyte production. (A) Schematic representation of the myl7:Tbx20 transgene used for inducible and cardiomyocyte-specific overexpression of Tbx20. The induction of Tbx20 in myl7-expressing cells is controlled by treatment with TBF (tebufenozide). (B–D) Representative confocal projections of myl7:nucGFP hearts from TBF-treated control embryos (B), non-TBF-treated myl7:Tbx20 injected embryos (C) and TBF-treated myl7:Tbx20-injected embryos (D) at 2 dpf. (E) Graph of the average number of nucGFP-positive cardiomyocytes in TBF-treated controls, non-TBF-treated myl7:Tbx20-injected embryos and TBF-treated myl7:Tbx20-injected embryos. Cardiomyocyte-specific overexpression of Tbx20 increased cardiomyocyte production. Error bars indicate standard deviations. ns indicates no significant difference compared with control embryos (n=6, p>0.05). Asterisks indicate a significant increase compared with control embryos (n=6, ***p<0.001). EXPRESSION / LABELING:
|
|
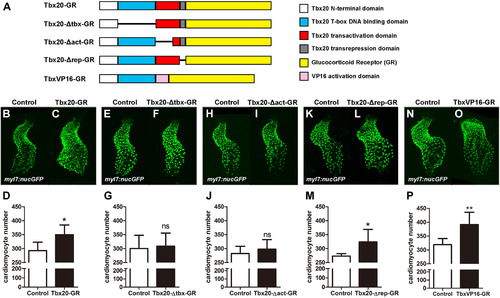
Tbx20's cardiac expansion activity requires its DNA binding and transcriptional activation domains. (A) Schematic diagram of the Tbx20-GR, Tbx20-Δtbx-GR (a T-box-defective form of Tbx20), Tbx20-Δact-GR (a transactivation-defective form of Tbx20), Tbx20-Δrep-GR (a transrepression-defective form of Tbx20), and TbxVP16-GR (a transactivation-only form of Tbx20) fusion proteins. (B–D) Tbx20-GR-injected embryos treated with DXS have significantly more cardiomyocytes (n=6). (E–G) Tbx20-Δtbx-GR overexpression did not significantly enhance cardiomyocyte production (n=6). (H–J) Overexpression of Tbx20-Δact-GR did not significantly enhance cardiomyocyte production (n=6). (K–M) Overexpression of Tbx20-Δrep-GR significantly increased cardiomyocyte number (n=6). (N–P) Overexpression of TbxVP16-GR significantly increased cardiomyocyte production (n=6). Error bars indicate standard deviations. ns indicates there is no significant difference compared with control embryos. Asterisks indicate a significant difference (*p<0.05, **p<0.01, ***p<0.001). EXPRESSION / LABELING:
|
|
LA1110 mutants exhibit severe pericardial edema. (A and B) Lateral views of two-day-old wild type (A) and LA1110 mutant (B) embryos. (C and D) Lateral views of wild type (C) and LA1110 mutant (D) embryos at day 3 of embryonic development. PHENOTYPE:
|
|
Cardiac cell formation defects in LA1110 mutant embryos. (A–D) In LA1110 mutant embryos, the expression domains of tbx5a (B) and bmp4 (D) are strongly diminished at the 12-somite and 16-somite stages compared to wild type siblings (A, C). White arrowheads point to the expression domains of tbx5a (A, B) and bmp4 (C, D) within the heart-forming region. |
|
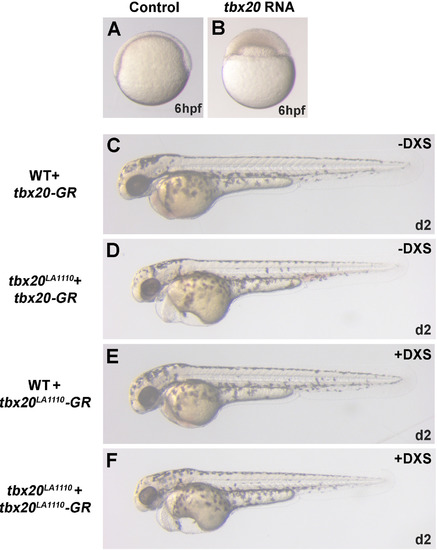
Tbx20 and Tbx20-GR overexpression phenotypes. (A and B) Uninjected control embryos (A) have begun to gastrulate by 6 h post fertilization, but overexpression of Tbx20 by injection of tbx20 mRNA disrupts gastrulation (B). (C and D) In the absence of DXS, 23.5% (12 out of 51 embryos from the tbx20LA1110 heterozygous crosses) of tbx20-GR injected embryos showed a characteristic tbx20LA1110 cardiac edema phenotype (D), demonstrating a failure to rescue without DXS induction. The remainder of the embryos displayed normal morphology (C). (E and F) Tbx20LA1110-GR overexpression in the presence of DXS induction did not rescue tbx20LA1110 mutants or induce developmental abnormalities, as 27.6% (16 out of 58 embryos from the tbx20LA1110 heterozygous crosses) of the embryos displayed the characteristic tbx20LA1110 cardiac edema phenotype (F) while the remainder were morphologically normal (E). |
|
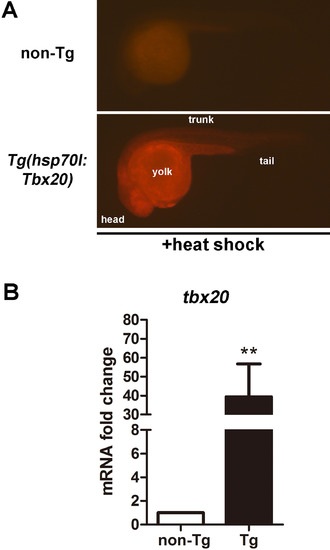
Tbx20 overexpression in the Tg(hsp70l:Tbx20) line. (A) mCherry fluorescence was observed in hsp70l:Tbx20 embryos after heat shock. The head, trunk, tail and yolk domains of the embryo are labeled. (B) Quantitative RT-PCR analyses demonstrated an approximately 40-fold increase in tbx20 expression hsp70l:Tbx20 embryos after heat shock. |
Reprinted from Developmental Biology, 421(2), Lu, F., Langenbacher, A., Chen, J.N., Tbx20 drives cardiac progenitor formation and cardiomyocyte proliferation in zebrafish, 139-148, Copyright (2017) with permission from Elsevier. Full text @ Dev. Biol.