- Title
-
Six1 regulates proliferation of Pax7-positive muscle progenitors in zebrafish
- Authors
- Nord, H., Skalman, L.N., and von Hofsten, J.
- Source
- Full text @ J. Cell Sci.
|
six1a and six1b are expressed in fast muscle progenitors. (A,B) Whole mount in situ hybridization showing non-overlapping expression of (A) six1a (blue) and smyhc1 (red) and of (B) six1b (blue) and smyhc1 (red), in 12-ss, 18-ss and 24-hpf embryos. Cross-sections are shown below or as an inset; lines indicate the levels of cross-sections. Brackets at 12ss indicate areas with strongest six1 expression. Dashed lines at 12ss indicate somitic borders, black arrowheads indicate expression in the rostral part of the somite and white arrowheads indicate expression in the caudal part of the somite. Scale bars: 100μm. EXPRESSION / LABELING:
|
|
six1 morphants have a reduced fast muscle area. (A) Ventral and lateral views of head (left two columns) and lateral view and cross-section of the trunk (right two columns) of 3-dpf tg(mylz2:GFP)i135 embryos uninjected and injected with six1a and six1b morpholinos alone or in combination. Cross-sections are stained with F310. (B) F310+ cross-section area of 24-hpf and 3-dpf embryos; the area of uninjected control embryos is set as 100%. Compared with control embryos at 24hpf (n = 5), six1a morphants have an area of 94.0% (n = 5), six1b morphants 88.4% (n = 5) and six1a and six1b double morphants 92.8% (n = 5). In 3-dpf embryos compared with control embryos, (n = 5) six1a morphants have an area of 84.9% (n = 5), six1b morphants 55.0% (n = 5) and six1a/six1b double morphants 37.2% (n = 6). Compared with uninjected control embryos (n = 4) set as 100%, six1a/p53 morphants have an F310+ cross-section area of 79.7% (n = 4), six1b/p53 morphant embryos 62.1% (n = 5) and six1a/six1b/p53 triple morphants have an area of 55.6% (n = 4). (C) Average number of DAPI-stained nuclei per F310+ (fast muscle specific) cross-section area of 3-dpf embryos. Uninjected control embryos have an average of 151.3 DAPI-stained nuclei per F310+ cross-section area (n = 5), six1a morphants have 156.0 (n = 5), six1b morphants 135.2 (n = 6) and six1a/six1b double morphants 119.5 (n = 7). 3-dpf six1a/p53 morphants have an average number of 156 DAPI-stained nuclei per F310+ cross-section area (n = 4), six1b/p53 morphants 152.2 (n = 5), six1a/six1b/p53 triple morphants 119.3 (n = 4) and control embryos have 159.5 (n = 4). (D) Number of slow fibres per somite in 24-hpf and 3-dpf embryos. fb, fin bud; hh, hyohyoideus; ih, interhyoideus; ima, intermandibular anterior; imp, intermandibular posterior; om, ocular muscles; sh, sternohyoideus; som, somites. Cross-sections were made at somite number 10 for 24-hpf embryos, and at somite number 8 for 3-dpf embryos. *P<0.05, **P<0.01, ***P<0.001. Scale bars: 50μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Expression pattern of six1b:GFPJ1. (A) Expression of Pax7 (red) and GFP (green) in cross-sections at 12 and 15ss and lateral view of somite number 2–3 at 12, 15 and 18ss. (B) GFP expression of six1b:GFP at 24hpf phenocopying mRNA expression of six1b. (C) Cross-section at somite number 10 and lateral view of six1b:GFP (green) stained with Pax7 (red); arrowheads indicate coexpression. (D,E) GFP expression in lateral view of whole embryo; dorsal (top) and ventral (bottom) view of head; and cross-section at somite number 8 and enlargement of trunk of six1b:GFP embryos at 48hpf (D) and 4dpf (E). AP, adenohypophyseal placode; LLN, lateral line neuromasts; LLP, lateral line placode; NC, notochord; NT, neural tube; Olf.P, olfactory placode; Olf.V, olfactory vesicle; OV, otic vesicle; SH, sternohyoideus; Som, somites; PF, pectoral fin. Scale bars: 100μm. EXPRESSION / LABELING:
|
|
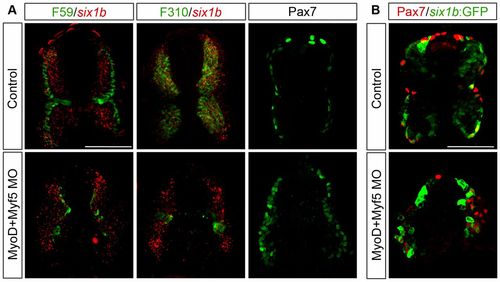
six1b expression in Pax7+ cells is independent of MyoD and Myf5. (A) Cross-section of uninjected control embryos and myoD/myf5 morphant embryos at 24hpf showing mRNA expression of six1b (red) together with slow muscle marker F59 (green) or fast muscle marker F310 (green) and of Pax7 alone. six1b expression in the Pax7+ domain remains in MyoD/Myf5 double morphants. (B) Cross-section of control and myoD/myf5 morphant six1b:GFP embryos at 24hpf showing expression of Pax7 (red) together with six1b:GFP (green). six1b:GFP and Pax7 expression domains coincide in the myoD/myf5 morphants. Cross-sections were made at somite number 10. Scale bars: 50 μm. |
|
six1b expression in Pax7+ cells is independent of MyoD and Myf5. (A) Cross-section of uninjected control embryos and myoD/myf5 morphant embryos at 24hpf showing mRNA expression of six1b (red) together with slow muscle marker F59 (green) or fast muscle marker F310 (green) and of Pax7 alone. six1b expression in the Pax7+ domain remains in MyoD/Myf5 double morphants. (B) Cross-section of control and myoD/myf5 morphant six1b:GFP embryos at 24hpf showing expression of Pax7 (red) together with six1b:GFP (green). six1b:GFP and Pax7 expression domains coincide in the myoD/myf5 morphants. Cross-sections were made at somite number 10. Scale bars: 50μm. |
|
Overexpression of six1a and six1b promotes Pax7+ cell proliferation and inhibits fibre differentiation. (A) Average number of Pax7+ cells per somite in control embryos (n = 18) and embryos injected with six1a (n = 19) or six1b RNA (n = 17) at 24hpf. Control embryos have 42.7 Pax7+ cells/somite whereas the number is increased to 47.4 and 49.2 Pax7+ cells/somite after overexpressing six1a and six1b RNA, respectively. (B) Proportions of GFP+ fibres and GFP+ non-differentiated myotomal cells in 18-ss embryos after injecting a heat-shock-inducible construct driving the expression of gfp, either alone or together with six1a or six1b coding sequences, induced at 70% epiboly. The control GFP construct has 42.7% non-differentiated myotomal cells and 57.3% fibres (n = 6); induction of six1a expression results in 89.0% non-differentiated myotomal cells and only 11.0% fibres (n = 4); six1b overexpression gives a proportion of 95.6% non-differentiated myotomal cells and 4.4% fibres (n = 4). Somite number 8–10 was counted. (C) Expression of GFP (green) and Pax7 (red) in 18-ss embryos after inducing expression of gfp alone or together with six1a or six1b coding sequences at 70% epiboly. Yellow arrowheads indicate GFP+/Pax7+ cells. (D) Expression of GFP and tdTomato at 16ss in fast-fibre-specific myosin light chain 2:GFP, tg(mylz2:GFP)i135 embryos injected with a heat-shock-inducible construct driving the expression of tdtomato alone or together with six1a or six1b coding sequences, induced at 70% epiboly. Overexpressed six1a or six1b both resulted in a decreased occurrence of fibre-like morphology in the induced cells. Yellow arrowheads indicate GFP+/tdTomato+ fibres, white arrowheads indicate fibres only expressing tdTomato. *P<0.05, ***P<0.001. Scale bars: 50μm. |
|
Six1 is required for pSmad1/5/8 expression in Pax7+ cells in the peripheral dermomyotome and reduced levels of pSmad1/5/8 decrease the number of Pax7+ cells. (A) Expression of Pax7 (red) and pSmad1/5/8 (green) in 24-hpf embryos uninjected and injected with six1a and six1b morpholinos alone or in combination. (B) Average number of Pax7+/pSmad1/5/8- and Pax7+/pSmad1/5/8+ cells per somite in control embryos (n = 6) and in six1a (n = 14), six1b (n = 11) and six1a and six1b double morphants (n = 13). Somite number 10-12 was counted. (C) Cross-sections of 24-hpf control embryos and six1a, six1b and six1a/six1b morphant embryos stained with Pax7 (red), pSmad1/5/8 (green) and DAPI (blue). Enlargements of Pax7+/pSmad1/5/8+ border are presented, with arrowheads indicating coexpression. (D) Expression of six1b (blue) and smyhc1 (red) in embryos treated with DMSO or 15µM dorsomorphin (DM) from shield stage to 24hpf, showing a maintained six1b expression; dashed lines indicate the level of cross-section. (E) Pax7 expression in embryos treated with DMSO or 5µM dorsomorphin from 2–3hpf to 24hpf. (F) Pax7 expression in embryos treated with 100 or 200µM dorsomorphin from 10ss to 24hpf. (G) Embryos treated with 5µM dorsomorphin from 2–3hpf to 24hpf have an average number of 32.8 Pax7+ cells/somite (n = 24) and DMSO-treated controls have 46.7 Pax7+ cells/somite (n = 18). Embryos treated with 100µM dorsomorphin from 10ss to 24hpf have an average number of 33.1 Pax7+ cells/somite (n = 19) and DMSO-treated controls have 41.4 Pax7+ cells/somite (n = 17). Embryos treated with 200µM dorsomorphin from 10ss to 24hpf have an average number of 34.8 Pax7+ cells/somite (n = 19) and DMSO-treated control have 41.3 Pax7+ cells/somite (n = 16). Somite number 10–12 was counted and cross-sections were made at somite number 10. *P<0.05, **P<0.01, ***P<0.001. Scale bars: 50μm and 10μm for enlargements. |
|
The six1a morpholino targets the six1a exon1/intron1 splice junction. (A) Complementary sequence of six1a cDNA of control embryos and embryos injected with six1a morpholino, dashed line indicates premature splice site. (B) Control six1a cDNA and cDNA from six1a morpholino injected embryos run on an agarose gel shows a smaller product in the morphant embryos. (C) Brightfield (left) and fluorescent (right) pictures showing six1b:GFP embryos uninjected and injected with six1a ATG morpholino, illustrating the efficiency of the morpholino. |
|
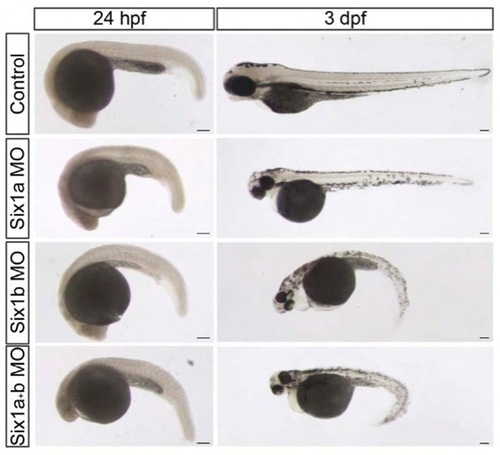
six1 morphants have a trunk phenotype. Brightfield pictures of control embryos, six1a, six1b and six1a/six1b morphant embryos at 24 hpf and 3 dpf. Scale bars: 100 μm. |
|
Six1 is not sufficient to induce pSmad1/5/8 expression in Pax7+ cells. Expression of Pax7 (blue), GFP (green) and pSmad1/5/8 (red) in 24-hpf embryos injected with a heat shock inducible construct driving the expression of gfp together with six1a or six1b coding sequence. Arrowheads indicate Pax7+/GFP+ cells. Scale bar: 50 μm. |