- Title
-
Eaf1 and Eaf2 negatively regulate canonical Wnt/β-catenin signaling
- Authors
- Liu, J.X., Zhang, D., Xie, X., Ouyang, G., Liu, X., Sun, Y., and Xiao, W.
- Source
- Full text @ Development
|
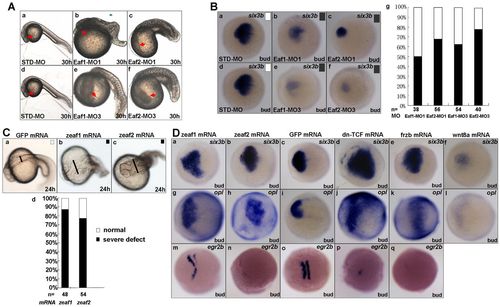
Effects of eaf1 and eaf2 knockdown and overexpression on zebrafish embryos. (A) Morphology of representative embryos injected with the indicated MOs (8 ng/embryo) at 30 hpf. STD-MO, standard MO. (B) (a-f) In situ hybridization for the anterior brain marker six3b in the presence of the indicated MOs. (g) Scoring of six3b expression: black, reduced expression; white, normal expression. (C) (a-c) Morphology of representative embryos injected with the indicated mRNAs (200-500 pg/embryo). zeaf1 and zeaf2 refer to zebrafish eaf1 and eaf2. (d) Morphology was scored as normal (white) or severely defective (black) in eaf1- and eaf2-overexpression embryos. The expanded dorsal part is indicated by the black line. (D) eaf1 and eaf2 function as forebrain inducers in embryos. Embryos injected with eaf1 and eaf2 mRNA displayed increased expression of six3b (a,b) and opl (g,h), similar to embryos injected with dn-Tcf (d,j) or frzb (e,k) mRNA, but in contrast to embryos injected with wnt8a mRNA (f,l); egr2b exhibited reduced expression in embryos injected with eaf1 and eaf2 mRNA (m,n), similar to embryos injected with dn-Tcf (p) or frzb (q) mRNA. Ba-f,Da-q, dorsal views, anterior to the left. |
|
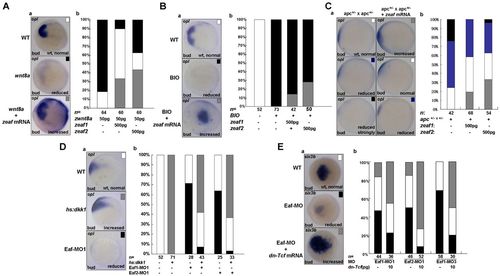
Eafs antagonize Wnt/β-catenin signaling in anterior neuroectoderm patterning. (A) eaf1 and eaf2 counteract wnt8a-mediated forebrain defects. (a) In situ hybridization for the anterior neuroectoderm marker opl in wild type (WT) and in the presence of wnt8a mRNA alone or together with eaf1 or eaf2 mRNA. (b) Scoring of opl expression: black, reduced; white, normal; gray, expanded. (B) eaf1 or eaf2 partially counteracts the strong reduction in opl expression that occurs after exposure to the Gsk3 inhibitor BIO, which enhances Wnt/β-catenin signaling. opl expression is scored as in A. (C) eaf1 and eaf2 rescue forebrain defects in apc mutants. opl expression is scored as: black, strongly reduced; blue, reduced; white, normal; gray, increased. (D) Heat shock-induced expression of dkk1 at 50% epiboly rescues forebrain defects in Eaf morphants. opl expression is scored as in A. (E) dn-Tcf partially rescues the anterior brain defects in Eaf morphants. Embryos were scored by six3b expression: black, reduced; white, normal; gray, increased. Aa,Ba,Ea, dorsal views, anterior to the left; Ca,Da, lateral views, anterior to the left. |
|
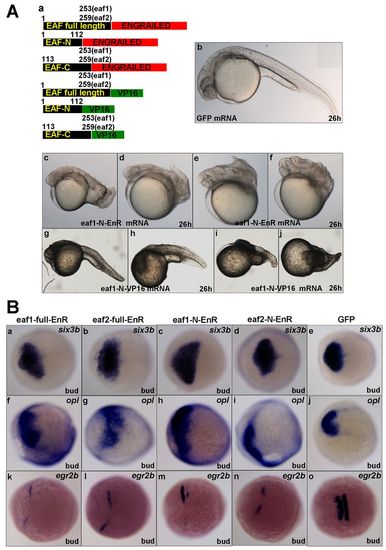
Zebrafish eaf1 and eaf2 induce anterior neuroectoderm by acting as transcriptional repressors. (A) (a) mRNAs used for injection encode full-length or the N- or C-terminus of Eaf1 or Eaf2 fused with the transcriptional activator VP16 or the engrailed transcriptional repressor domain (EnR). Numbers refer to amino acid residues in Eaf1 and Eaf2. (b) Embryos injected with GFP mRNA at 26 hpf exhibit normal morphology. (c-f) Embryos injected with eaf1-N-EnR mRNA display similar morphology to embryos injected with full-length eaf1 mRNA. (g-j) Embryos injected with eaf1-N-VP16 mRNA display phenotypes with obvious anterior neuroectoderm truncation, similar to Eaf morphants. (B) eaf1 and eaf2 act as anterior neuroectoderm inducers by repressing transcription. six3b (a-e) and opl (f-j) displayed expanded expression in embryos injected with eaf1/2-EnR or eaf1/2-N-EnR mRNA. (k-o) egr2b expression was reduced in embryos injected with eaf1/2-EnR or eaf1/2-N-EnR mRNA. Ba-o, dorsal views, anterior to the left. All mRNA injections were at 50 pg/embryo. |
|
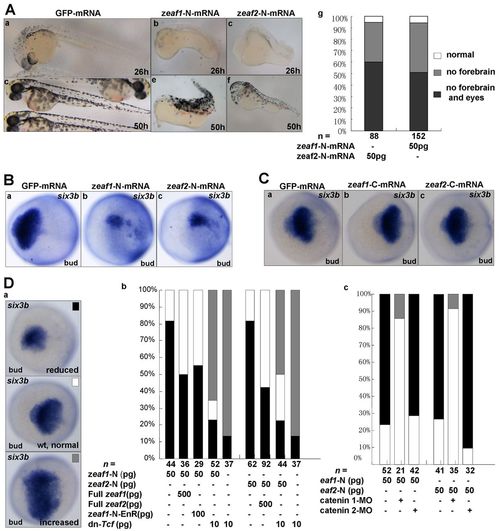
The N-terminus of Eaf1 or Eaf2 acts as a dominant negative. (A) (a-f) Representative morphology of embryos injected with GFP mRNA or mRNA encoding the N-terminus of Eaf1 or Eaf2 (exons 1-3) at 26 or 50 hpf. (g) Embryos were scored morphologically at 50 hpf: white, the percentage of normal embryos; gray, embryos without forebrain; black, embryos without forebrain and eyes. (B,C) six3b expression was reduced in embryos injected with mRNA encoding the N-terminus (exons 1-3) of Eaf1 or Eaf2 (B) but was normal in embryos injected with mRNA encoding the C-terminus (exons 4-6) of Eaf1 or Eaf2 (C). (D) The N-terminus (exons 1-3) of Eaf1/2 suppresses forebrain formation by promoting enhanced Wnt/β-catenin activity. (a) Representative embryos showing normal, reduced and increased expression of six3b. (b) Embryos were scored for six3b expression after injection with mRNAs at the dosages indicated. (c) Abnormal expression of six3b in embryos injected with mRNA encoding the N-terminus (exons 1-3) of Eaf1 or Eaf2 was rescued by co-injection with β-catenin1-MO (8 ng/embryo) but not with β-catenin2-MO (8 ng/embryo). Ba-c,Ca-c,Da, dorsal views, anterior to the left. mRNA injections were at 50 pg/embryo unless stated otherwise (Db). |
|
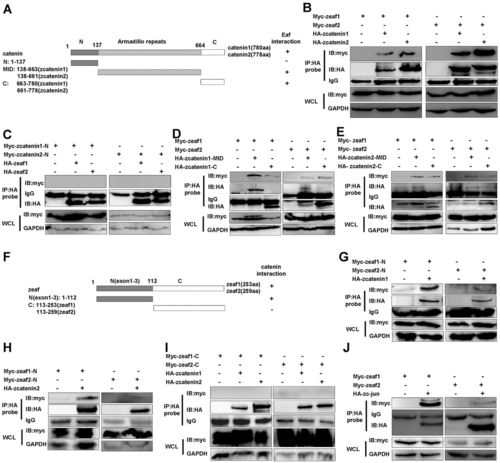
Eaf1 and Eaf2 function as novel nuclear Wnt signaling components. (A) The domains in zebrafish β-catenin 1 and β-catenin 2 that interact with Eaf1 and Eaf2: +, interaction; –, no interaction. MID, the β-catenin center region that contains 12 Armadillo repeats. (B) Co-immunoprecipitation of Eaf1 or Eaf2 with full-length β-catenin 1 or β-catenin 2. (C) Eaf1 or Eaf2 did not interact with the N-terminus of β-catenin 1 or β-catenin 2. (D,E) Eaf1 or Eaf2 interacted with both the Armadillo repeats and the C-terminal transcriptional domain of β-catenin 1 (D) and β-catenin 2 (E). (F) The domains in Eaf1 and Eaf2 that interacted with β-catenin 1/2: N, exons 1-3 of Eaf1 or Eaf2; C, exons 4-6 of Eaf1 or Eaf2; +, interaction; –, no interaction. (G,H) β-catenin 1 (G) and β-catenin 2 (H) interacted with the N-terminus of Eaf1 or Eaf2. (I) β-catenin 1/2 did not interact with the C-terminus of Eaf1 or Eaf2. (J) Co-immunoprecipitation of Eaf1 or Eaf2 with full-length zebrafish c-Jun. WCL, whole cell lysate. |
|
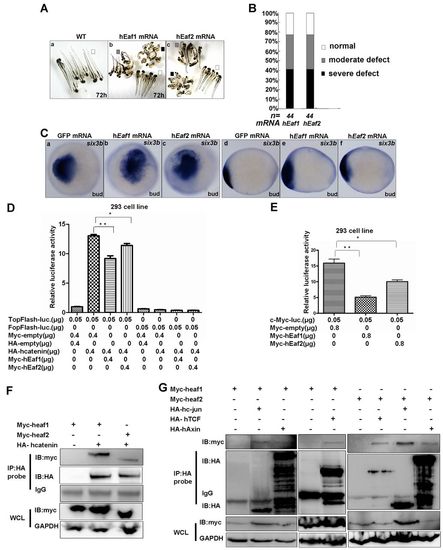
Eaf1/2 activity is evolutionarily conserved from zebrafish to human. (A) Phenotypes of zebrafish embryos injected with human EAF1 (hEaf1) or EAF2 (hEaf2) mRNA at 72 hpf. (B) Morphology of zebrafish embryos injected with human EAF1 or EAF2 mRNA at 3 dpf (200-500 pg/embryo) was scored as normal (white), moderately defective (gray) or severely defective (black); embryos injected with GFP mRNA (200-500 pg/embryo) were used as a control. (C) six3b displayed enhanced expression in zebrafish embryos in which human EAF1 or EAF2 was ectopically expressed. (D) 8xTopFlash activity enhanced by human β-catenin was suppressed in the HEK 293 cell line by human EAF1 or EAF2. (E) Downregulation of c-MYC promoter activity by human full-length EAF1 or EAF2. Mean±s.d.; **P<0.01, *P<0.05 (Student’s t-test). (F) Co-immunoprecipitation of human EAF1 or EAF2 with full-length human β-catenin. (G) Human EAF1 and EAF2 interact with components of the human β-catenin transcriptional complex, including TCF4, c-JUN and AXIN, in vivo. Ca-c, dorsal views, anterior to the left; Cd-f, lateral views, anterior to the left. WCL, whole cell lysate. |
|
The expression patterns of zebrafish eaf1 and eaf2 during embryogenesis. (A) eaf1 expression. (B) eaf2 expression. Lateral view, dorsal to the right. EXPRESSION / LABELING:
|
|
Validation of Eaf1-MO3 and Eaf2-MO3. (A) (a) Schematic of eaf1 exon-intron structure, Eaf1-MO3 targeting position and RT-PCR primer locations. (b) In STD-MO injection embryos, an expected 333 bp band could not be detected by RT-PCR, but in embryos with Eaf1-MO3 injection, a 333 bp band could be detected by RT-PCR. (B) (a) Schematic of eaf2 exon-intron structure, Eaf2-MO3 targeting position and RT-PCR primer locations. (b) A 420 bp band was amplified from embryos injected with STD-MO by RT-PCR, but a 892 bp band was amplified from embryos injected with Eaf2- MO3, which contained intron 1 of eaf2. Embryos were collected at the bud stage for RNA extraction. Lane 1 is RNA from embryos injected with STD-MO, and lane 2 is RNA from embryos injected with Eaf1-MO3 (Ab) or Eaf1-MO3 (Bb). |
|
Knockdown of zebrafish Eaf1 and Eaf2 causes defects in anterior neuroectoderm patterning and mesoderm patterning. (A) The expression of anterior neuroectoderm marker foxg1. (B-D) The expression of dorsal mesoderm marker genes sqt and gsc. (E-H) The expression of posterior ectoderm/mesoderm marker cdx4 and ventral mesoderm marker tbx6. (A) Dorsal view, anterior to the left; (D) dorsal view; (B,C) animal view, dorsal to the right; (E-H) lateral view, dorsal to the right. |
|
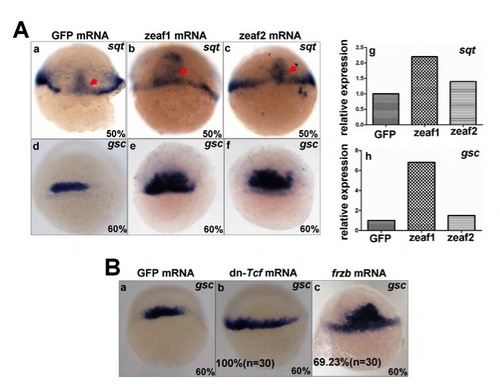
Ectopic expression of zebrafish Eaf1 and Eaf2 increases the expression of sqt and gsc. (A) At the gastrula stage, dorsal marker genes sqt (a-c) and gsc (d-f) displayed expanded expression, particularly in the ventral domain. qRTPCR was used to measure expression of sqt (g) and gsc (h) in these embryos. (B) In embryos injected with dn-Tcf mRNA (b) or frzb mRNA (c), the expression of gsc expanded ventral-laterally at the gastrula stage, compared with embryos injected with GFP mRNA (a). Dorsal views. |
|
eaf1 and eaf2 antagonize Wnt/β-catenin signaling. (A) The expression of axin2, a bona fide direct target of Wnt/β-catenin signaling at bud stage, was enhanced after knockdown of eaf1 (b,e) or eaf2 (c,f) (red arrows). (B) The offspring of apc+/- × apc+/- were genotyped by sequencing the mutant allele DNA fragment amplified from genomic DNA extracted from WISH-stained embryos. The embryos with normal opl expression contain the wild-type allele (C/C) (a,d), whereas the embryos with reduced opl expression contain the heterozygous allele (C/T) (b,e) and the embryos with strongly reduced opl expression contain the homozygous mutated allele (T/T) (c,f). (C) β-catenin1-MO, but not β-catenin2-MO, partially rescued the anterior brain defects in Eaf morphants. (D) At the 30% gastrula stage, β-catenin2- MO partially reduced the increased gsc expression associated with Eaf morphants (a-h); both β-catenin1-MO and β-catenin2-MO partially reduced the increased gsc expression of Eaf2-MO1 morphants at the sphere stage (i-m), and β-catenin2-MO was more effective (m). (A-C) Dorsal view, anterior to the left; (Da-g,i-l) dorsal view. |
|
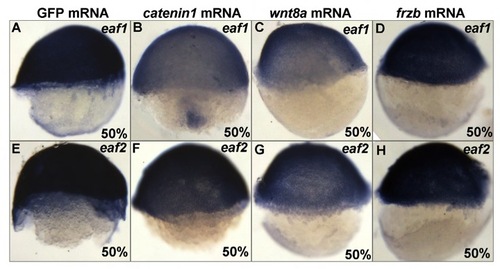
The regulation of zebrafish eaf1 and eaf2 by Wnt/β-catenin signaling. The expression of eaf1 and eaf2 is downregulated in embryos with ectopic expression of active β-catenin 1 mRNA (B,F) or wnt8a mRNA (C,G) as compared with embryos injected with GFP mRNA (A,E), but was maintained in embryos injected with frzb mRNA (D,H). Lateral views, dorsal to the right. |
|
VP16 and EnR are not functional in vivo and Eaf1/2 act as transcriptional repressors. (A,B) Six3b staining indicated normal patterning of anterior neural ectoderm in embryos injected with mRNA of the engrailed domain (EnR) or the VP16 domain (A), and in embryos injected with EnR or VP16 fused with the C-terminus of Eaf1 or Eaf2 (B). (C) In the embryos injected with eaf1/2-EnR or eaf1/2-N-EnR mRNA, the expression of the dorsal marker genes gsc and chd displayed similarly expanded patterns. (A,B) Dorsal views, anterior to the left; (C) animal views, dorsal to the right. All mRNA injections were 50-100 pg/embryo. |
|
Zebrafish Eaf1 or Eaf2 does not affect the protein level of either β-catenin 1 or β-catenin 2. (A) Western blot analysis for total β-catenin and active β-catenin protein (Ac-catenin) in embryos injected with STD-MO (lane 1), Eaf1- MO1 (lane 2), Eaf2-MO1 (lane 3), Eaf1-MO3 (lane 4) or Eaf2-MO3 (lane 5) (8 ng/per embryo). (B) The co-transfection of increased amounts of eaf1 or eaf2 together with β-catenin 1 and β-catenin 2 did not change the protein level of β-catenin 1 and β-catenin 2. (C) Western-blot analysis confirmed ectopic expression of zebrafish Eaf1 and Eaf2. |
|
Zebrafish Eaf1 or Eaf2 colocalizes with zebrafish β-catenin 1, β-catenin 2 and c-Jun. Cos-7 cells were transfected with: (A) empty GFP together with eaf1-RFP; (B) empty GFP together with eaf2-RFP; (C) empty RFP together with β-catenin1-GFP; (D) β-catenin1-GFP together with eaf1-RFP; (E) β-catenin1-GFP together with eaf2-RFP; (F) c-jun-GFP together with empty RFP; (G) c-jun-GFP together with eaf1-RFP; (H) c-jun-GFP together with eaf2-RFP; (I) β-catenin2-GFP together with empty RFP; (J) β-catenin2-GFP together with eaf1-RFP; (K) β-catenin2-GFP together with eaf2-RFP. The cells were photographed under a fluorescence microscope 24 hours after transfection. |
|
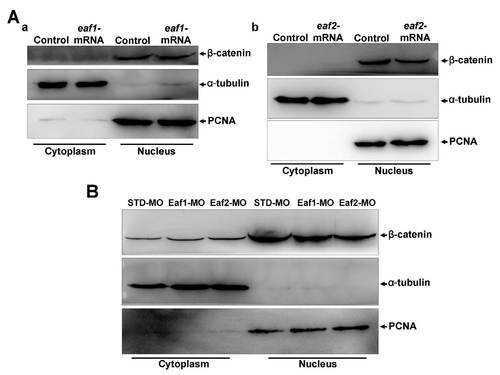
Ectopic expression or knockdown of zebrafish eaf1 or eaf2 in embryos at the gastrula stage does not change cytoplasmic-nuclear translocation of β-catenin. (A) Ectopic expression of eaf1 (a) or eaf2 (b) did not change cytoplasmic-nuclear translocation of β-catenin. (B) Knockdown of eaf1 or eaf2 did not change cytoplasmic-nuclear translocation of β-catenin. |
|
Human Eaf function is evolutionary conserved in regulating Wnt/β-catenin signaling. (A,B) In zebrafish embryos injected with human EAF1 or EAF2 mRNA, dkk1 (A) and frzb (B) displayed enhanced expression. (C) Overexpression of human EAF1 or EAF2 did not affect co-transfected human β-catenin stability. (D) Overexpression of human EAF1 or EAF2 did not change the endogenous active β-catenin protein level. (E-G) Ectopic expression of human TCF4 (E), human c-JUN (F) and human AXIN (G) after co-transfection with human EAF1 or EAF2 into the HEK 293T cell line. |