- Title
-
Zebrafish ift57, ift88, and ift172 intraflagellar transport mutants disrupt cilia but do not affect hedgehog signaling
- Authors
- Lunt, S.C., Haynes, T., and Perkins, B.D.
- Source
- Full text @ Dev. Dyn.
|
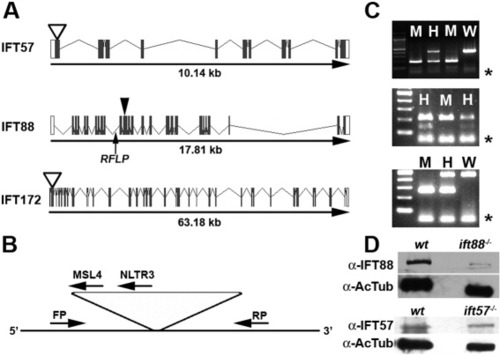
Zebrafish IFT gene structure and location of mutations. A: Gene structure of the zebrafish ift57, ift88, and ift172 genes is shown with exons as solid boxes. The 5′-UTR and 3′-UTR regions are shown as white boxes. ift57 and ift172 mutants resulted from retroviral insertional mutagenesis (Sun et al.,[2004]) and the insertion site is shown as an open arrowhead. The causative mutation for ift88 was identified in exon 11 (Tsujikawa and Malicki,[2004]), which is shown by a closed arrowhead. An arrow shows the location of the intragenic RFLP used for genotyping ift88 mutants. B: Generic diagram for genotyping ift57 and ift172 insertional mutants. PCR was performed with a three-primer combination of a forward primer (FP), a reverse primer (RP), and a viral-specific primer (MSL4 or NLTR3). Due to the size of the viral insert (>6 kb), the FP and RP product only amplified from the wild type allele. If a mutant allele was present, the FP and a viral-specific primer generated a PCR product. When analyzed by agarose gel electrophoresis, homozygous embryos produce a single band, whereas heterozygous embryos produced bands from both alleles. C: Typical examples of genotyping analysis for ift57 (top), ift88 (middle), and ift172 (bottom) embryos. Homozygous ift57 mutants (M) produced a single 210-bp band, whereas reactions from heterozygous embryos (H) produced the mutant band and a 448-bp wild type band (W). The RFLP in the ift88 gene can be digested by Bcl I on the wild type allele but fails to cut on the mutant allele. ift88 mutant embryos (M) were identified by the absence of a lower band following Bcl I digestion, as previously described (Tsujikawa and Malicki,[2004]). Following PCR and Bcl I digestion, heterozygous animals (H) retained both the cut and uncut products. Homozygous ift172 embryos produced a single band of 265 bp, whereas reactions from heterozygous embryos produced the mutant band and a 461-bp band. Asterisks (*) denote excess primer at the bottom of the gel. D: Western blot analysis of 48-hpf embryos. Extracts of wild type (wt) and mutant embryos (ift88-/- or ift57-/-) were immunoblotted for either IFT88 or IFT57 protein. Acetylated tubulin (AcTub) was used as a loading control. |
|
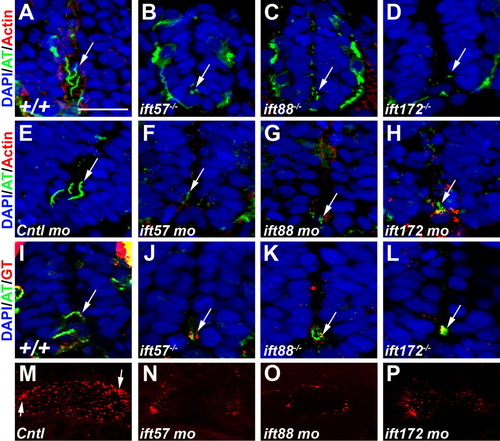
Loss of IFT disrupts ciliogenesis. A-D: Acetylated tubulin antibody (green) labeled primary cilia extending from cells in the ventral neural tube of 24-hpf wild type embryos. E-H: Cilia were disrupted in ift57, ift88, and ift172 mutant embryos at 24 hpf. Embryos injected with a control morpholino show cilia. Cilia were disrupted in 24-hpf embryos following injection of morpholinos against IFT genes. In morphant embryos, stabilized microtubules were still observed in small puncta that likely correspond to the basal body region. Arrows point to regions of acetylated tubulin staining. Rhodamine phalloidin (red) labels actin filaments. I-L: Gamma tubulin (red) recognized basal bodies that colocalized with acetylated tubulin (green) in the mutant embryos. In all sections nuclei are visualized using the nuclear stain DAPI. M-P: Fluorescent whole-mount images of the otic vesicle stained with acetylated tubulin at 18 hpf. Control embryos show clusters of longer cilia at the anterior and posterior edges (arrows) and numerous short cilia throughout the lumen. Cilia within the lumen are highly reduced or absent in IFT morphant embryos. The otic vesicles are also slightly smaller than control embryos. Scale bar = 20 μm for A-L. EXPRESSION / LABELING:
PHENOTYPE:
|
|
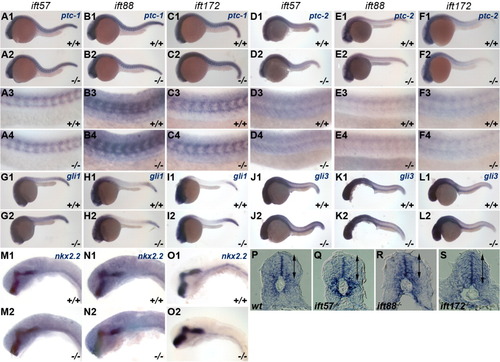
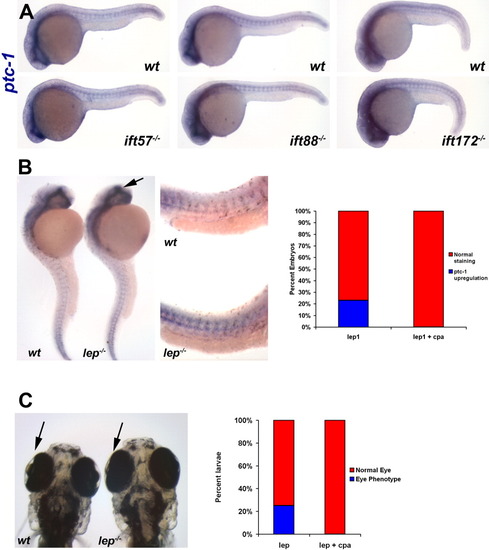
Expression of Hh target genes is unchanged in IFT mutants. In situ hybridization was performed for Hh target genes at 24 hpf. Embryos were photographed and subsequently genotyped. Wild type (+/+) and mutant (-/-) embryos are shown in individual panels for ift57 (A, D, G, J, M), ift88 (B, E, H, K, N), and ift172 (C, F, I, L, O) genotypes. A-C: Expression of ptc-1 and (D-F) ptc-2 remained unchanged in IFT mutants. A3-F4: High-magnification images of wild type and mutant embryos. G-I: gli1 and (J-L) gli3 remains unchanged at 24 hpf in wild type and IFT mutant embryos. M-O: High-magnification images of nkx2.2 staining in the ventral midbrain and hindbrain in wild type and IFT mutant embryos revealed no differences in expression. A1-O2: All images show lateral view of embryos, with anterior to the left. P-S: Sections of the neural tube from wild type and mutant embryos following in situ hybridization with ptc-1 reveal no differences in the dorsal or ventral expression boundaries. Arrows indicate the dorsal and ventral limits of ptc-1 expression. EXPRESSION / LABELING:
|
|
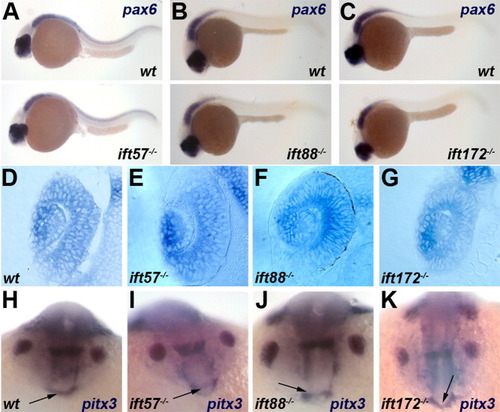
Expression of pax6 and pitx3 is unchanged in IFT mutants. A-C: Embryos at 24 hpf were examined for pax6 expression in the eye and neural tube. All images show lateral view of embryos, with anterior to the left. D-G: Transverse sections of wild type and genotyped IFT mutant retinas following in situ hybridization for pax6 transcripts at 24 hpf. H-K: Expression of the anterior pituitary marker pitx3 is unchanged in IFT mutants at 18 hpf. Arrows point to the pituitary anlage. EXPRESSION / LABELING:
|
|
Expression of shh and ptc-1 at 48 hpf is unchanged in IFT mutants. Lateral views of (A-C) shh expression and (D-F) ptc-1 in wild type (top) and IFT mutant (bottom) embryos. Dorsal view of (A′-C′) shh expression and (D′-F′) ptc-1 in wild type (left) and IFT mutant (right) embryos. Expression of both shh and ptc-1 in the pectoral fins (arrowheads) is unaltered. EXPRESSION / LABELING:
|
|
Alcian blue staining of jaw and brachial arch structure at 5 dpf. A-C: Wild type embryos raised in DMSO vehicle exhibit normal craniofacial head skeleton. D-L: IFT mutants displayed no reduction or loss of the major head structures and no fusion of the trabeculae. M-O: detourgli1 mutants showed significant narrowing of ethmoid plate (black arrow). P: Embryos treated with 100 μM cyclopamine lack all major head cartilage. The top row shows whole-mount images viewed from the ventral side. The middle row shows flat-mount images of dissected dorsal neurocranium structures. The bottom row shows flat-mounted images of dissected ventral structures to show brachial arches. PHENOTYPE:
|
|
A low dose of cyclopamine does not affect Hh signaling in IFT mutants. A: Expression of ptc-1 is unaffected in 24-hpf embryos following addition of 5 μM cyclopamine to progeny of IFT heterozygous incrosses. At least 30 embryos were examined from each incross. Two representative embryos from each clutch are shown. Images show a lateral view of embryos, with anterior to the left. B: In situ hybridization experiment demonstrating that ptc-1 expression is increased in lep mutants. Arrow shows increased ptc-1 in the forebrain of lep mutants. High magnification reveals increased ptc-1 expression in the trunk of lep1 mutants (middle panel). Quantification of phenotypes are shown in the graph. C: At 5 dpf, the lens degeneration in lep mutants can be observed. The graph shows that addition of cyclopamine rescues this phenotype. |
|
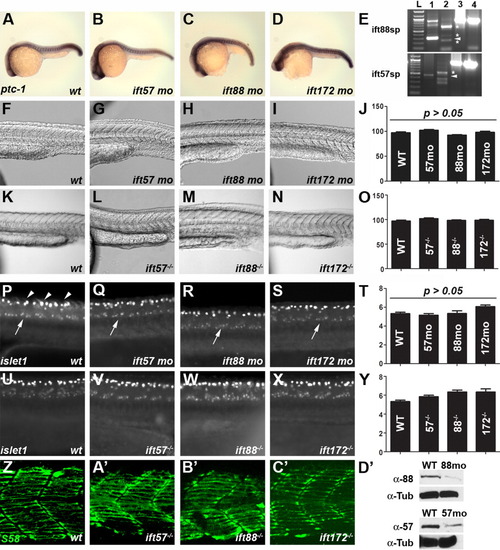
Loss of IFT function does not affect somite formation or spinal motor neuron development. A-D: Expression of ptc-1 in wild type and IFT morphants is unchanged at 24 hpf. In situ hybridization was done simultaneously and embryos were developed for the same amount of time. At least 20 embryos were examined for each treatment. E: RT-PCR of embryos injected with ift88 (top) or ift57 (bottom) splice-blocking morpholinos. Lane 1 is an uninjected control and shows the wild type transcript, as indicated by the arrowhead in each panel. Lane 2 is RT-PCR from a morpholino injected animal at 72 hpf. Aberrant splice products are denoted by asterisks and no wild type product is present. Injection of ift88-sp morpholinos caused the inclusion of intron 4-5 (340 bp) and resulted in a longer amplification product as the major band. Two minor products likely reflect loss of exon 4 (60 bp) and an aberrant splice product. Injection of ift57-sp morpholinos results in the inclusion of intron 2-3 (88 bp) and results in a longer amplification product and introduces a stop codon. Two smaller amplification products were also observed. All products are predicted to produce frame-shifts and introduce a stop codon. Lanes 3 and 4 show amplification of beta-actin from cDNA used in lanes 1 and 2, respectively. F-O: Brightfield images using Nomarski optics reveal no defects in somites in wild type and IFT morphants or mutants. Somites form the characteristic chevron shape in all IFT morphant and mutant embryos. Quantification of somite angles (J, O) revealed no statistical difference between wild type and IFT morphants or mutants. P-Y: Whole-mount immunocytochemistry with the islet-1 antibody labels motor neurons in the ventral spinal cord (arrows). Clusters of 5-7 positively-stained neurons were typically seen on each side of the embryos. These neurons are found in similar numbers among wild type, IFT mutant, or IFT morphants embryos (T, Y). This antibody also strongly labels Rohon-Beard sensory neurons in the dorsal spinal cord (arrowheads). Z-C′: S58 staining labeled slow-muscle fibers in the chevron-shaped somites of both wild type and genotyped mutant embryos at 24 hpf. Some muscle fibers stained with S58 were below the plane of optical sectioning and not contained in these images. D′: Western blot analysis of 18-hpf embryos injected with IFT morpholinos. Extracts of wild type (wt) and morphant embryos (88mo or 57mo) were immunoblotted for either IFT88 or IFT57 protein. Alpha tubulin (α-Tub) was used as a loading control. |