- Title
-
A two-step model for colon adenoma initiation and progression caused by APC loss
- Authors
- Phelps, R.A., Chidester, S., Dehghanizadeh, S., Phelps, J., Sandoval, I.T., Rai, K., Broadbent, T., Sarkar, S., Burt, R.W., and Jones, D.A.
- Source
- Full text @ Cell
|
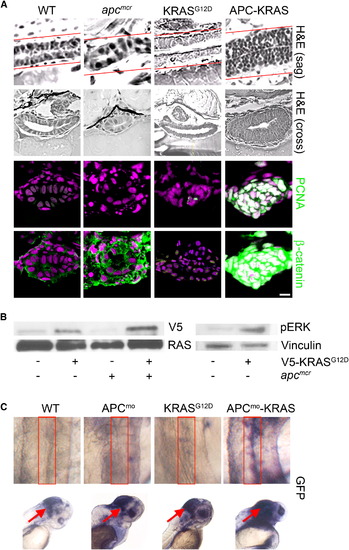
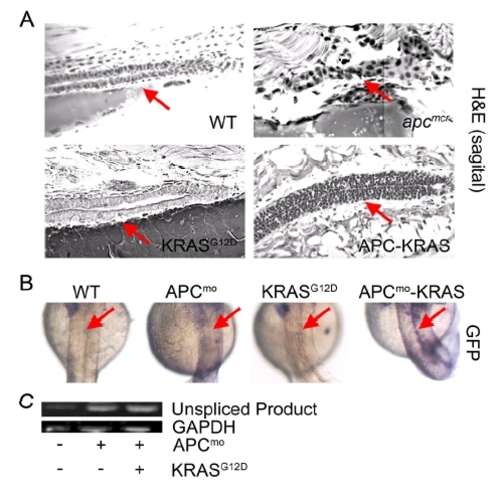
KRAS Promotes Intestinal Cell Proliferation and Nuclear Localization of β-Catenin following Loss of APC (A) apcmcr zebrafish embryos were injected at the one-cell stage with KRAS mRNA. The 72 hpf embryos were fixed, sectioned, and stained for hematoxylin and eosin (H&E) (row 1, sagital section; row 2, cross section), DNA (magenta, middle and bottom), and either PCNA (middle, green) or β-catenin (bottom, green). Overlapping expression is shown in white. (B) Protein lysates from 72 hpf embryos were subjected to western blot analysis for either V5-tag (top-left) and total RAS (bottom-left) or phospho-Erk (top-right) and vinculin (bottom-right). (C) Zebrafish harboring an integrated β-catenin TOPGFP reporter were injected with KRAS mRNA, APC morpholino, or both. The 72 hpf embryos were subjected to whole-mount in situ hybridization for GFP. Boxes indicate the intestine (top), and arrows indicate the hindbrain (bottom). All images were captured using the same exposure and represent at least three independent experiments. Scale bar, 10 μm. EXPRESSION / LABELING:
|
|
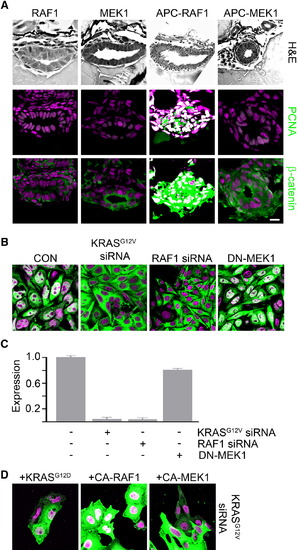
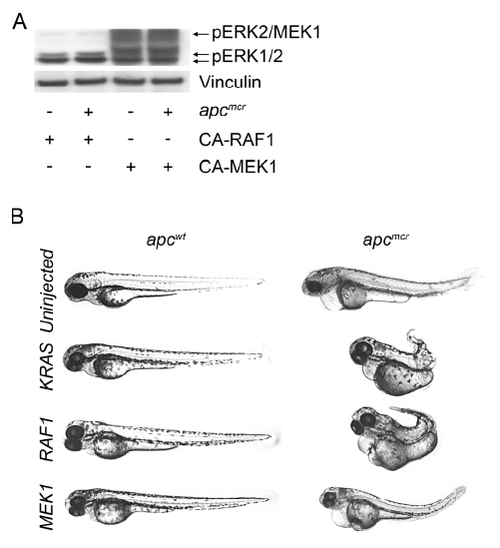
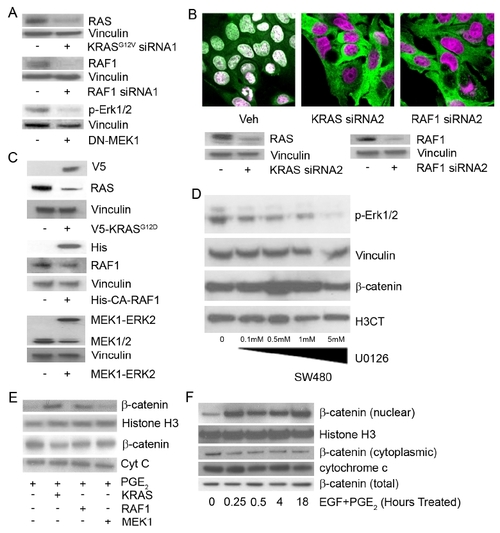
KRAS and RAF1 Are Necessary for Nuclear Localization of β-Catenin and Intestinal Cell Proliferation following Loss of APC (A) apcmcr zebrafish embryos injected with constitutively active RAF1 or MEK1. The 72 hpf embryos were stained for hematoxylin and eosin (H&E) staining (top), DNA (magenta, middle and bottom), and either PCNA (middle, green) or β-catenin (bottom, green). (B) SW-480 cells transfected with control, KRASG12V, RAF1-directed siRNA, or DN-MEK1 constructs were stained for DNA (magenta) and β-catenin (green). (C) RNA was harvested and subjected to rt-PCR for Axin2. Error bars, SEM. (D) SW-480 cells transfected with KRASG12V-specific siRNA were cotransfected with constitutively active KRAS, RAF1, or MEK1 and stained for DNA (magenta) and β-catenin (green). Overlapping expression is shown in white. All images were captured using the same exposure and represent three independent experiments. Scale bar, 10 μm. |
|
KRAS and RAF1 Direct Stabilized β-Catenin to the Nucleus (A) WT and KRAS-injected zebrafish embryos treated with PGE2 were stained for DNA (magenta), PCNA (green), and β-catenin (green). (B) Human 293 cells were transfected with constitutively active KRAS, RAF1, or MEK1 and either DMSO (top) or PGE2 (bottom). Cells were stained for DNA (magenta) and β-catenin (green). (C) 293 cells treated with DMSO, PGE2 or PGE2, and leptomycin B were stained for DNA (magenta) and β-catenin (green). (D) 293 cells were transfected with control or KRAS- or RAF1-directed siRNA and treated with EGF and PGE2 or vehicle and then stained for DNA (magenta) and β-catenin (green). (E) 293 cells were subjected to an MTT assay (*p < 0.05 versus DMSO; error bars, SEM). Overlapping expression is shown in white. All images were captured using the same exposure and represent three independent experiments. Scale bar, 10 μm. |
|
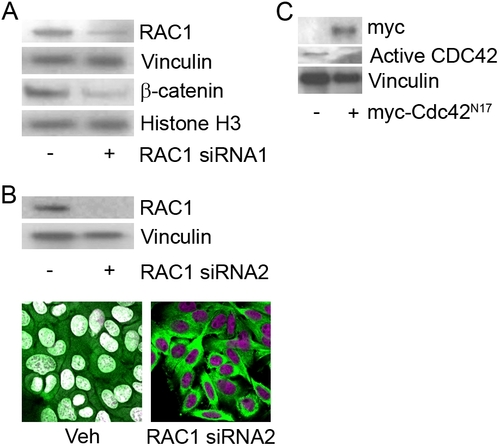
KRAS/RAF1 Regulation of β-Catenin Requires the Activity of RAC1 (A and B) SW-480 cells transfected with vehicle or myc-tagged ([A], S191A or S605A) or flag-tagged ([B], S552A or S675A) β-catenin mutants were stained for DNA (magenta) and α-myc ([A], green) or α-flag ([B], green). (C) SW-480 cells were transfected with RAC1-directed siRNA, DN-RAC1, and DN-Cdc42 or treated with the RAC1-specific inhibitor NSC23766 and stained for DNA (magenta) and β-catenin (green). (D) SW-480 cells were subjected to an MTT assay (*p < 0.05 versus DMSO; error bars, SEM). (E) SW-480 cells were transfected with vehicle, KRASG12V-targeted siRNA, RAF1 siRNA, or DN-MEK1 and subjected to a RAC1 activity assay. The western blot was probed for RAC1 (top). Control lysates were probed for total RAC1 (bottom). (F) SW-480 cells treated as above were stained for phospho-cJun. (G) Human 293 cells transfected with constitutively active RAC1 and treated with PGE2 or DMSO were stained for DNA (magenta) and β-catenin (green). All images were captured using the same exposure and are representative of at least three independent experiments. Scale bar, 10 μm. |
|
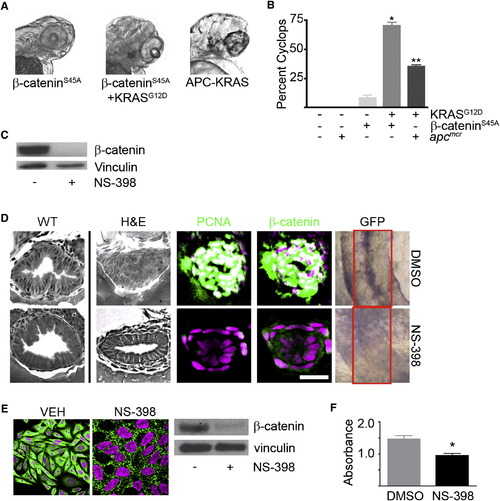
KRAS-Mediated Intestinal Cell Proliferation following Loss of APC Requires β-Catenin (A) Zebrafish embryos were injected with β-cateninS45A mRNA (left) along with KRAS mRNA (middle). Also shown is a representative image of the APC-KRAS embryo (right). At 72 hpf, the embryos were fixed and photographed. (B) The percent cyclops was analyzed (*p < 0.01 WT versus β-catenin-KRAS, **p < 0.01 WT versus APC-KRAS). (C) Protein was harvested from apcmcr embryos treated with DMSO or NS-398 and subjected to western blot analysis for β-catenin (top) or vinculin (bottom). (D) Wild-type uninjected or apcmcr embryos injected with KRAS mRNA treated with VEH (top) or NS-398 (bottom) were stained by H&E (WT, left; APC-KRAS, right) and for DNA (magenta), PCNA (green), and β-catenin (green). TOPGFP-APCmo-KRAS embryos were stained for GFP expression (purple). Boxes indicate the intestine. (E) SW-480 cells treated with DMSO or NS-398 were stained for DNA (magenta) and β-catenin (green). Protein lysates were subjected to western blot analysis for β-catenin (top) and vinculin (bottom). (F) Cells were subjected to MTT analysis (*p < 0.05 versus DMSO; error bars, SEM). Overlapping expression is shown in white. All images were captured using the same exposure and represent at least three independent experiments. Scale bar, 10 μm. |
|
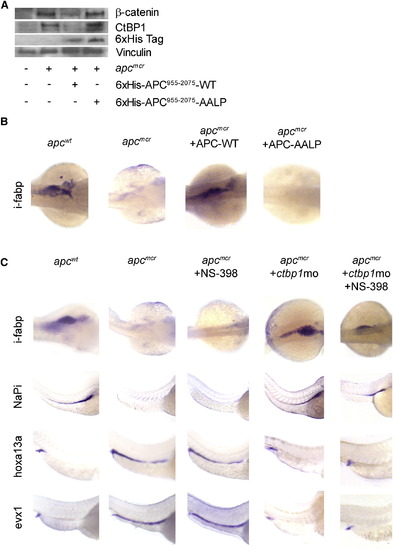
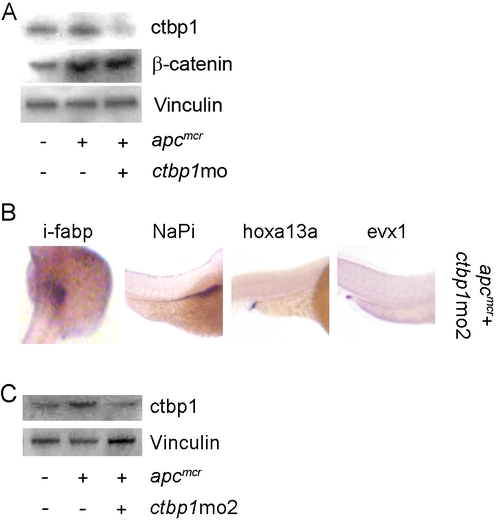
apc Control of Cellular Fate and Differentiation Are Mediated by ctbp1 and Are β-Catenin Independent (A) Protein from 48 hpf apcmcr zebrafish embryos injected with either 6 x His-APC955–2075 or 6 x His-APC955–2075-AALP were subjected to western blot analysis for β-catenin (top), ctbp1 (second), 6 x His (third), or vinculin (bottom). (B) Embryos injected as above were fixed at 72 hpf and subjected to in situ hybridization for i-fabp. (C) apcmcr embryos were treated with DMSO or NS-398 or injected with ctbp1-directed morpholino in the presence or absence of NS-398. At 72 hpf, the embryos were fixed and subjected to in situ hybridization for i-fabp, NaPi, hoxa13a, or evx1. All images are representative of at least three independent experiments. EXPRESSION / LABELING:
|
|
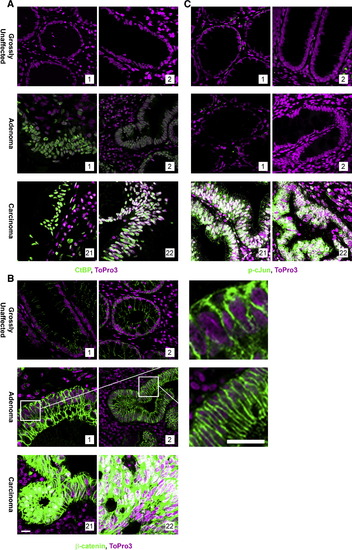
JNK1 Activation Is Coincident with Nuclear Accumulation of β-Catenin in Human Tumor Samples FFPE human-matched grossly uninvolved and adenoma samples obtained from FAP patients and unmatched sporadic carcinomas were stained to indicate DNA (magenta) and (A) CtBP (green), (B) β-catenin* (green), or (C) phospho-cJun (green) as an indicator of JNK activity. Overall, 20 patients matched grossly uninvolved, and adenoma or unmatched carcinoma tissue samples were stained. Shown are two representative samples. All images were captured using the same exposure, and overlapping expression is shown in white. *Note: A section from each of the β-catenin-stained adenomas was enlarged to the right. Each antibody was evaluated using serial sections. Scale bar, 5 μm. |
|
KRAS Promotes Intestinal Cell Proliferation and Activation of β- Catenin following Loss of apc |
|
APC-KRAS and APC-RAF1 Share Morphologic Abnormalities that Are Distinct from APC-MEK1 Embryos |
|
KRAS/RAF1 and COX-2/PGE2 Are Required to Maintain Nuclear Localization of β-Catenin in Human Cells |
|
RAC1, but Not Cdc42, Activity Is Necessary for Nuclear Localization of β-Catenin in SW-480 Cells |
|
ctbp1 Knockdown Does Not Affect β-Catenin Levels |
Reprinted from Cell, 137(4), Phelps, R.A., Chidester, S., Dehghanizadeh, S., Phelps, J., Sandoval, I.T., Rai, K., Broadbent, T., Sarkar, S., Burt, R.W., and Jones, D.A., A two-step model for colon adenoma initiation and progression caused by APC loss, 623-634, Copyright (2009) with permission from Elsevier. Full text @ Cell