- Title
-
Short- and long-range functions of Goosecoid in zebrafish axis formation are independent of Chordin, Noggin 1 and Follistatin-like 1b
- Authors
- Dixon Fox, M., and Bruce, A.E.
- Source
- Full text @ Development
|
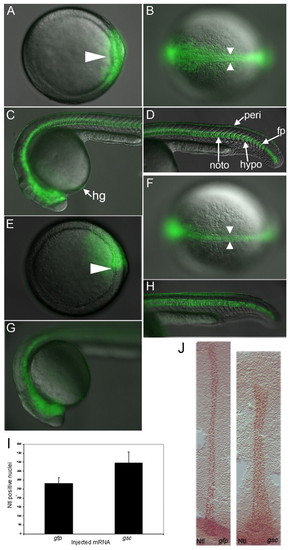
Ventral gsc overexpression induces complete secondary axes. (A-I) Live zebrafish embryo injected with gfp (A-C) or gsc (D-I) RNA. (A-F,H,I) DIC and fluorescence merge; (G) DIC. (A,D) Shield (arrowhead), animal view. (B,F) Bud; arrowheads indicate notochord, asterisk indicates prechordal plate. (C) 1 dpf, side view. (E) Bud; side view, secondary prechordal plate (asterisk). (G-K) 1 dpf, dorsal view. (J,K) dlx2a and shha (red). (J) gfp RNA injected. (K) gsc RNA injected showing duplicated dlx2a expression (brackets): staining differs from the control because the embryo had a ventral third eye. (L) Schematic of injection procedure, clones in green; one clone was generated per embryo. dien, diencephalon; e, eye; fp, floor plate; hg, hatching gland; myo, myotomes; noto, notochord; tele, telencephalon. |
|
Dorsal gsc overexpression induces excess dorsal tissue. (A-H) Merged images of live zebrafish embryo injected with gfp (A-D) or gsc (E-H) RNA. (A,E) Shield (arrowhead). (B,F) Bud, arrowheads indicate notochord. (C,D,G,H) 1 dpf. (I) Notochord cell counts with standard deviations, five embryos per treatment. (J) Bud, anterior to the top. Anti-Ntl staining is brown, injected RNA is listed in lower right. hypo, hypochord; peri, periderm. |
|
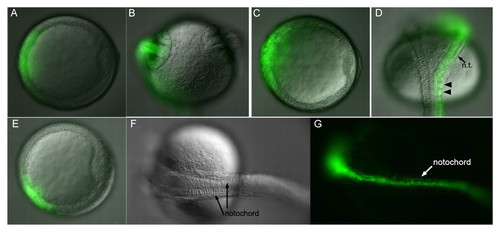
gsc recruits unlabeled cells to secondary axes. (A-K) Zebrafish embryos injected with gsc RNA. (A-C) Lateral views, (A) bud; (B,C) 1 dpf. (D,E) Animal views; (F-K) dorsal views, 1 dpf. (A,B,F,I) DIC; (G,J) fluorescence; (C,H,K) merge. (A) Secondary notochord with somites (arrowheads) at bud. (B,C) Myotomes (arrowheads) with GFP-labeled notochord (dashed lines). (D,E) Shield stage gsc RNA-injected embryos stained for membrane-GFP (brown) and chd (purple), with ectopic chd (arrowheads). (F-H) Secondary neural tube with GFP-labeled (arrow) and unlabeled cells; arrowhead marks anterior GFP limit. (I-K) Low gsc RNA dose ventral clone produces partial secondary axis. (K) Arrows indicate unlabeled cells in neural tissue (right inset) and in myotomes (left inset). (L-O) Shield (arrowheads). (L,N) gfp RNA injected; (M,N) gsc RNA injected, with wnt8 (L,M) and chd (N,O) in situ probe. nt, neural tissue. |
|
gsc induces secondary axes in the absence of Chd. (A-G) Live chdtt250 embryos injected ventrally with 24 pg (A-D) or 48 pg (E-G) gsc RNA. (A,C,E) Shield, dorsal to the right. (B,D) 1 dpf, anterior to the top. (F,G) 1 dpf, anterior to the left. (B) Secondary axis with head (2/7, 29%). (D) Secondary axis without notochord (5/7, 71%). (F,G) Two notochords (arrows, 9/12, 75%). nt, neural tissue. |
|
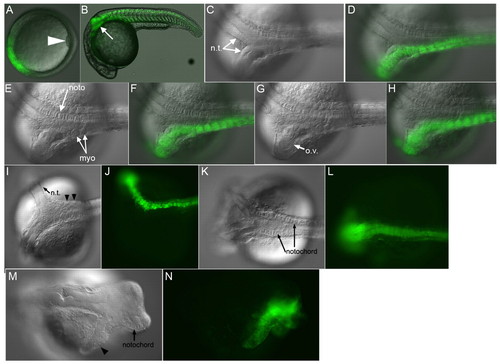
chd induces partial secondary axes. (A-H) Live zebrafish embryo injected with chd RNA. Shield (A), 1 dpf (B-H). (B) Partial secondary axis (arrow, 13/21, 62%). GFP-labeled neural tissue (C,D) (13/13, 100%), myotomes (E,F) (9/13, 69%) and ectopic otic vesicle (G,H) (6/13, 46%). Some injected embryos had beating cardiac tissue (4/13, 31%). (I-L) Live chdtt250 embryos injected ventrally with 48 pg gsc and nog1- and fstl1b-MOs. (I,J) Partial GFP-labeled secondary axis with neural (arrow) and somitic (arrowheads) tissue. (K,L) Secondary axis containing GFP-labeled notochord and neural tissue (3/5, 60%). For an example of an uninjected chd mutant embryo see Fig. 7D. (M,N) Wild-type embryo injected ventrally with chd, fstl1b and nog1 mRNAs. Arrowhead marks neural tissue. ov, otic vesicle. |
|
gsc elicits distinct short- and long-range signaling activities. Injected construct is listed in lower left. (A-D,H,I) Live embryos at 1 dpf. (A,B) Embryo with two notochords (arrows). (C,D) Embryo with partial secondary axis, with neural (arrow) and somitic (arrowheads) tissue. (E-G) Shield stage embryos stained for chd. (E) Control; (F,G) gsc-VP2-injected embryos. Arrowheads indicate ectopic chd. (H,I) Dorsalized embryo with partial secondary axis. |
|
gsc and chd morpholinos affect DV patterning. Injected construct or genotype is listed in lower right. (A-H) Live embryos, 1 dpf. (I-P) Tails, 1 dpf; stained with anti-Ntl antibody, anterior to the left. Arrowheads indicate thin notochords, arrows indicate truncated notochords. (Q-S) 60% epiboly embryos stained for wnt8; arrowhead marks dorsal. (Q) Control. (R) gsc-MO-injected embryo with ectopic wnt8 expression dorsally (11/81, 14%). (S) Ectopic staining in embryo injected with gsc-MO and a low concentration of chd-MO (46/106, 43%). |
|
Time-lapse of dorsal clones. (A-H) Still images of dorsolateral clones, lateral views. Arrowheads indicate dorsal midline and arrows indicate notochord boundaries. (A-D) gfp RNA-injected control embryo from shield (A) to 90% (D). (E-H) gsc RNA-injected embryo from 60% epiboly (E) to 2-somite stage (H). |
|
chd does not induce gsc. Animal views of shield (arrowhead) stage embryos stained for gsc and injected with (A) gfp (n=18) or (B) chd RNA (n=26). |
|
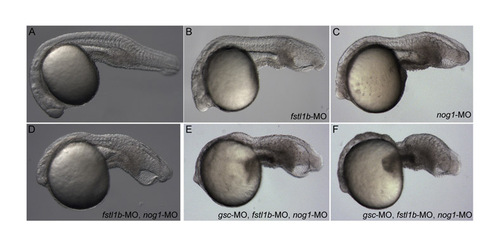
Morpholinos. (A-F) Live chdtt250 mutant embryos at 1 dpf; injected construct is listed in lower right. (A) control; (B) fstl1b-MO; (C) nog1-MO; (D) fstl1b- and nog1-MOs. (E,F) The most ventralized phenotypes seen in two examples of embryos injected with fstl1b-, nog1- and gsc-MOs. PHENOTYPE:
|
|
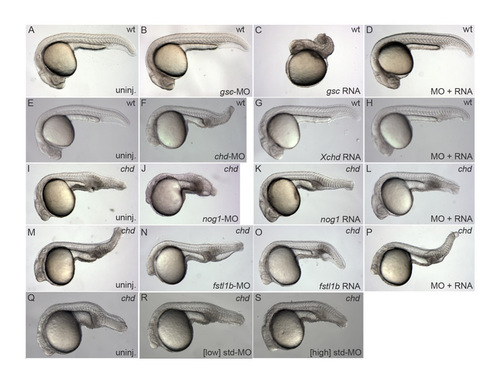
Morpholino controls. Live embryos at 1 dpf; genotype is listed in upper right, injected construct is listed in lower right. (A-D) gsc-MO blocks dorsalization by injected gsc, indicating that endogenous gsc is required for dorsalization. (E-G) chd-MO rescued with Xenopus chd RNA. (I-L) Rescue of nog1-MO by nog1 RNA. (M-P) Rescue of fstl1b-MO by fstl1b RNA. (Q-S) Neither low nor high concentrations of standard control morpholino (equivalent to single and triple morpholino experiments, respectively) enhance the chd mutant phenotype. |