- Title
-
Retinal homeobox 1 is required for retinal neurogenesis and photoreceptor differentiation in embryonic zebrafish
- Authors
- Nelson, S.M., Park, L., and Stenkamp, D.L.
- Source
- Full text @ Dev. Biol.
|
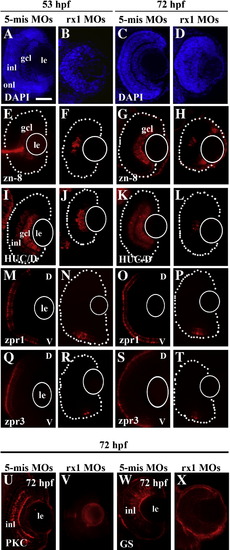
Morpholino-mediated depletion of rx1 results in lamination defects and reduced retinal cell differentiation. Embryos were treated at the 1–2 cell stage with a morpholino cocktail containing ATG (translational start site, 100 μM) and GT (splice donor site, 50 μM) directed morpholinos. (A–D) 5 μm sections stained with DAPI obtained at 53 hpf (A, B) and 72 hpf (C, D) from control (A, C) and rx-1-depleted (B, D) embryos, showing inhibition/delay of lamination in treated retinas. (E–H) Sectioned retinas stained for the ganglion cell surface marker, zn-8, evaluated at 53 hpf (E, F) and 72 hpf (G, H) from control (E, G) and rx1-depleted (F, H) retinas, showing patchy staining of the gcl as a consequence of treatment. (I–L) Sections stained for the amacrine and ganglion cell marker HuC/D, obtained at 53 hpf (I, J) and 72 hpf (K, L) from control (I, K) and rx1-depleted (J, L) embryos, showing reduced staining as a consequence of treatment. (M–P) Sections stained with the red/green double cone marker zpr1, from embryos fixed at 53 hpf (M, N) and 72 hpf (O, P) following treatment with control (M, O) and rx1-depleting (N, P) morpholinos, showing reduced cone differentiation in the treated retinas. (Q–T) Sections stained with the rod marker zpr3, from embryos fixed at 53 hpf (Q, R) and 72 hpf (S, T) following treatment with control (Q, S) and rx1-depleting (R, T) morpholinos, showing reduced rod differentiation in the treated retinas. (U–X) Sections stained for the rod bipolar cell marker, PCK (U, V) and the Müller glia cell marker GS (W, X) from control (U, W) and rx1-depleted (V, X) embryos fixed at 72 hpf, showing reduced differentiation of rod bipolar cells and the absence of Müller glia in treated retinas. le = lens; gcl = ganglion cell layer; inl = inner nuclear layer; onl = outer nuclear layer; V = ventral; D = dorsal; dotted lines indicate retinal boundary; white circles depict the location of the lens; scale bar = 50 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Capped rx1 mRNAs rescue the rx1 depletion phenotype. (A–C) Sectioned embryos processed for indirect immunofluorescence with zpr1 (cone marker), and counterstained with DAPI to reveal retinal lamination. Embryos were injected with 5-mispair control MOs (A), splice site-directed rx1-MOs (B), or splice site-directed rx1-MOs in combination with capped rx1 mRNAs, arrow indicates zpr1+ photoreceptors in ventral retina (C). le = lens; gcl = ganglion cell layer; inl = inner nuclear layer; onl = outer nuclear layer; V = ventral; D = dorsal; scale bar in A (applies to all) = 50 μm. |
|
Rx2 but not rx1 depletion causes a reduction in pax6 expression in optic primordia. (A–C) Embryos injected with MOs at the 1–2 cell stage and hybridized with a pax6 probe at 15 hpf. (A) 5-mispair control treated embryo (n = 48) showing the three regions of pax6 expression (arrowhead indicates anterior midline, arrow indicates eye anlage, asterisk indicates posterior midline). (B) rx1-depleted embryo showing similar pattern of expression of pax6 (n = 33). (C) rx2-depleted embryo (n = 24) showing a reduction in pax6 expression in the eye anlage and anterior midline. (D–F) Histograms showing the relative expression levels of pax6 in the eye anlage, anterior midline, and posterior midline, respectively, in 5-mispair control, rx1 and rx2 morphants. Scale bar in A (applies to all) = 100 μm. EXPRESSION / LABELING:
|
|
Expression of ath5 and pax6 but not rx2 is disrupted in rx1-depleted embryos. (A–C) Embryos fixed at 25 hpf and processed for in situ hybridization with an ath5 probe show strong retinal labeling in control embryos (A), no labeling in rx1-depleted embryos, and a normal ath5 expression pattern in rx2-depleted embryos. (D–F) Embryos fixed at 34 hpf and processed for in situ hybridization with a pax6 probe, showing labeling in the retina, lens and forebrain of control embryos (D), labeling in lens and forebrain only in rx1-depleted embryos (E), and a normal pattern of expression of pax6 in rx2-depleted embryos (F). (G–H) Sections obtained from embryos fixed at 53 hpf following injection with control-MOs (G) or rx1-depleting MOs (H) and hybridized with a probe for pax6, showing pax6 expression in the retinas of the morphants, albeit in a pattern reflecting a delay in neurogenesis. (I–L) Embryos processed at 34 hpf as whole mounts (I, J) or at 72 hpf as cryosections (K, L), for in situ hybridization with an rx2 probe, following injection with control-MOs (G) or rx1-depleting MOs, showing that rx2 is not regulated by rx1. le = lens; gcl = ganglion cell layer; inl = inner nuclear layer; onl = outer nuclear layer; cm = ciliary margin; scale bar in A and G = 50 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Rx1 morphants but not rx2 morphants exhibit significant changes in retinal progenitor proliferation. (A–H) Retinal cryosections stained with anti-PH3, a marker for cells in M-phase, from embryos fixed at 25 hpf (A, B), 34 hpf (C, D), 53 hpf (E, F), and 72 hpf (G, H), injected with control MOs (A, C, E, G) or rx1-depleting MOs (B, D, F, H). Staining is markedly reduced in the rx1 morphants at the early times, but increased at the later times; arrow in D indicates disorganized mitotic cells that were occasionally observed in the morphants. I. Average numbers of PH3-positive cells as a function of treatment and time of assessment. Significant differences were evident following rx1 depletion for all times evaluated (***p < 0.001) but rx2 depletion caused no changes. Scale bar = 50 μm; error bars indicate ± 1 SD. PHENOTYPE:
|
|
Rx1 and rx2 morphants exhibit significant increases in retinal cell death. (A–D) Grayscale images of live control (A, C) and rx1 morphant (B, D) embryos stained with acridine orange showing very little cell death at 25 hpf (A, B) but a dramatic increase in cell death (C vs. D) at 34 hpf in morphants as compared to controls; arrow in D indicates acridine orange-positive cells in the eye of a morphant. E. Average numbers of acridine orange-positive cells as a function of treatment and time of assessment. Significant differences were evident following rx1 and rx2 depletion when assessed at 34 hpf (rx1,***p < 0.001; rx2,**p < 0.01) but not at 25 hpf. (F–I) Grayscale images of sectioned control (F, H) and rx1 morphant (G, I) embryos processed for the TUNEL cell death assay at 53 hpf (F, G) and 72 hpf (H, I), showing increased cell death in rx1-depleted retinas; arrowheads in G and I indicate TUNEL-positive cells. (J) Average numbers of TUNEL-positive cells as a function of treatment and time of assessment. Significant differences were evident following rx1 depletion when assessed at 53 hpf and 72 hpf (***p < 0.001). le = lens; gcl = ganglion cell layer; inl = inner nuclear layer; onl = outer nuclear layer; scale bar in A (applies to A–D) and I (applies to F–I) = 50 μm; error bars indicate ± 1 SD. PHENOTYPE:
|
|
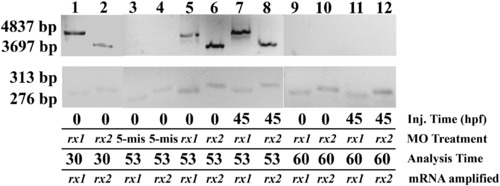
Efficacy and perdurance of rx1 and rx2 splice site-targeting MOs in embryos treated at the 1–2-cell stage (“0 hpf”) and at 44.5 hpf. The presence of large amplicons (upper row) indicates aberrant splicing. Lanes 1 and 2, embryos injected with rx1 or rx2-targeted MOs at 0 hpf and extracted at 30 hpf; lanes 3 and 4, embryos injected with the 5-mispair control MO at 0 hpf and extracted at 53 hpf; lanes 5 and 6, embryos injected with rx1 or rx2-targeted MOs at 0 hpf and extracted at 53 hpf; lanes 7 and 8, embryos injected with rx-1 or rx2-targeted MOs at 44.5 hpf and extracted at 53 hpf; lanes 9–12, embryos injected with rx1- or rx2-targeted MOs at 0 hpf (lanes 9 and 10) or at 44.5 hpf (lanes 11 and 12) and extracted at 60 hpf. |
|
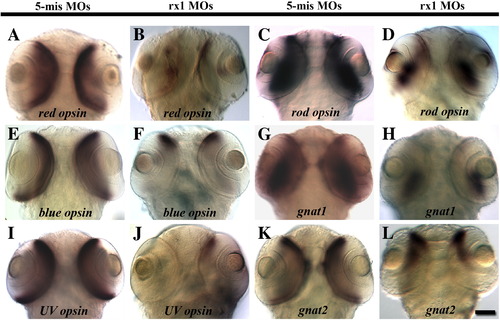
Temporally-selective rx1 depletion results in a reduction in the expression of rod and cone opsins and rod and cone transducins. Embryos were injected with the 5-mispair control MO (A, C, E, G, I, K) or an rx1-targeting MO cocktail (B, D, F, H, J, L) and were processed as whole mounts for in situ hybridization with cRNA probes corresponding to red cone opsin (A, B), rod opsin (C, D), blue cone opsin (E, F), rod transducin alpha subunit (gnat1; G, H), UV cone opsin (I, J), or cone transducin alpha subunit (gnat2; K, L). Scale bar = 50 μm. EXPRESSION / LABELING:
|
|
Temporally-selective rx2 depletion results in a selective reduction in the expression of rod and red cone opsins and rod and cone transducins. Embryos were injected with the 5-mispair control MO (A, C, E, G, I, K) or an rx2-targeting MO cocktail (B, D, F, H, J, L) and were processed as whole mounts for in situ hybridization with cRNA probes corresponding to red cone opsin (A, B), rod opsin (C, D), blue cone opsin (E, F), rod transducin alpha subunit (gnat1; G, H), UV cone opsin (I, J), or cone transducin alpha subunit (gnat2; K, L). Scale bar = 50 μm. EXPRESSION / LABELING:
|
|
Embryos subjected to temporally-selective (at 44.5 hpf) depletion of rx1 and rx2 show a significant reduction in photoreceptor differentiation. (A–B) DAPI-stained retinal cryosections obtained from control (A) and rx1/rx2-depleted (B) embryos fixed at 72 hpf; onl appears normal in both cases (arrow in B). (C–F) Retinal cryosections obtained from control (C, E) and rx1/rx2-depleted (D, F) embryos fixed at 72 hpf and hybridized with cRNA probes corresponding to NeuroD (C, D) and crx (E, F), showing no effect of retinal homeobox depletion on expression of these transcription factors. (G–J) Retinal cryosections obtained from control (G, I) and rx1/rx2-depleted (H,J) embryos fixed at 72 hpf and stained with the zpr1 (cones; G, H) and zpr3 (rods; I, J) antibodies, showing elimination of markers of photoreceptor terminal differentiation as a consequence of temporally-selective retinal homeobox knockdown. (K–M) Sections bisecting the lens were scored for the absence (‘none’) or presence of fewer than ten (‘few’) or more than ten (‘many’) zpr-1 or zpr3-stained photoreceptors. Relative numbers of sections displaying the indicate score are shown following temporally-selective depletion of rx1 (K), rx2 (L), and rx1 and rx2 (M). Knockdown of both rx1 and rx2 is more effective at reducing photoreceptor differentiation. le = lens, gcl = ganglion cell layer, inl = inner nuclear layer, onl = outer nuclear layer, scale bar in A = 50 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Cell proliferation related to the rod lineage is unaltered following temporally-selective depletion of rx1 and rx2. (A–B) Control (A) and rx-depleted (at 44.5 hpf; B) embryos were treated with BrdU at 60 hpf, and fixed at 72 hpf for BrdU indirect immunofluorescence; patterns of BrdU incorporation are similar; BrdU-positive cells residing between the dotted lines were used for the analysis shown in C. (C) Boxplot showing numbers of BrdU-positive cells as a function of treatment and laminar position. There are no significant differences between treated and control numbers. The boxes indicate the 25th and 75th percentiles, the lines indicate the median values, and the whiskers indicate the upper and lower limits. le = lens, gcl = ganglion cell layer, inl = inner nuclear layer, onl = outer nuclear layer, cm = ciliary margin, scale bar in A = 50 μm. |

Unillustrated author statements EXPRESSION / LABELING:
|
Reprinted from Developmental Biology, 328(1), Nelson, S.M., Park, L., and Stenkamp, D.L., Retinal homeobox 1 is required for retinal neurogenesis and photoreceptor differentiation in embryonic zebrafish, 24-39, Copyright (2009) with permission from Elsevier. Full text @ Dev. Biol.