- Title
-
Flamingo regulates epiboly and convergence/extension movements through cell cohesive and signalling functions during zebrafish gastrulation
- Authors
- Carreira-Barbosa, F., Kajita, M., Morel, V., Wada, H., Okamoto, H., Martinez Arias, A., Fujita, Y., Wilson, S.W., and Tada, M.
- Source
- Full text @ Development
|
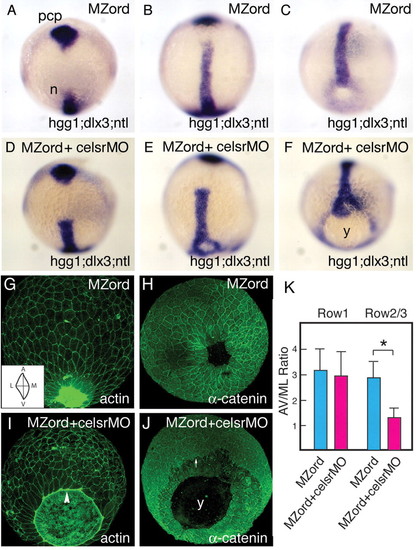
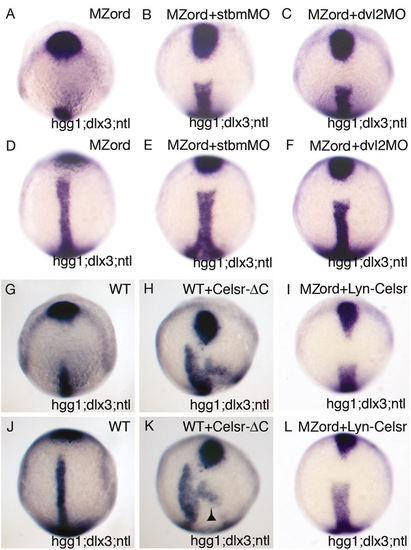
Injection of celsr1a and celsr1b morpholinos in MZord embryos leads to a defective epiboly phenotype during zebrafish gastrulation. MZord embryos (A,B,C,G,H) or MZord embryos injected with 0.4 pmoles each of celsr1a and celsr1b morpholinos (D,E,F,I,J) were visualised at tail-bud stage with markers, as indicated in the bottom right-hand corner, by in situ hybridisation. hgg1 was used to indicate the prechordal plate (pcp), ntl for the prospective notochord (n) and germ ring blastopore margin, and dlx3 for the anterior edge of the neural plate (A-F). Embryos were also visualised at 90% epiboly with phalloidin for actin (G,I) or anti-α-catenin antibody (H,J). An arrowhead represents the leading edge of the EVL with the actin cable being formed (I). An arrow indicates the leading edge of deep cells (J). Note that the deep cells are delayed from the leading edge of the enveloping layer in the mutant/morphant embryos. y, yolk. (K) Cell elongation of the EVL along the animal-vegetal (AV) axis and the mediolateral (ML) axis at 90% epiboly is quantified as AV/ML ratio (see the inset of G), and the measurement is made using ImageJ from 80 cells (four embryos of each group). Cells that are attached with the actin cable are expressed as `Row1', whereas cells that are not attached to that as `Row2/3'. Means and standard deviations (s.d.) are shown, and an asterisk indicates a statistically significant difference (P<0.05; Student's t-test). |
|
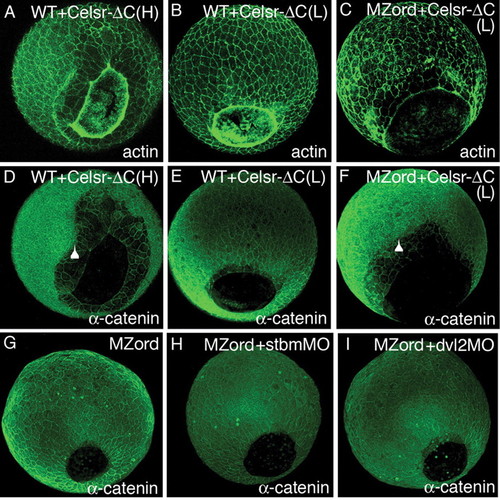
A C-terminal truncated form of Celsr2 (Celsr-ΔC) is dominant negative and Celrs regulates epiboly independently of the PCP. Wild-type (A,B,D,E) or MZord embryos (C,F) were injected with 300 pg (H, high) (A,D) or 150 pg (L, low) (B,C,E,F) RNA encoding Celsr-ΔC-HA were visualised at 90% epiboly with phalloidin for actin (A-C) or anti-α-catenin (D-F). Arrowheads indicate the leading edge of deep cells, showing epiboly defects with deep cells retracted from the leading edge of the EVL. (G-I) MZord embryos (G) or MZord embryos injected with 0.4 pmoles stbmMO (H) or 0.75 pmoles dvl2MO (I) were visualised at 90% epiboly with anti-α-catenin. Dorso-posterior views of 90% epiboly embryos. Anterior is upwards and the antibodies used are indicated in the bottom right-hand corner. MZord embryos injected with stbmMO or dvl2MO show normal epiboly movements, as assessed by closure of the germ ring blastopore. |
|
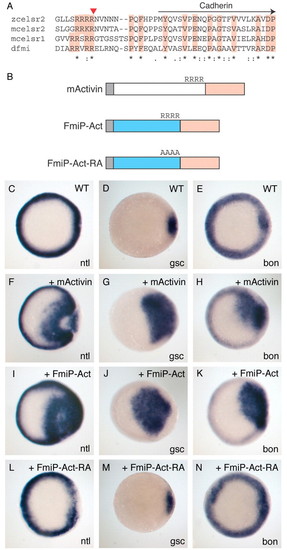
Celsr/Flamingo has a potential pro-region mediating dimer formation. (A) The comparison of the region N-terminal to the Cadherin repeats indicates a Furin-like protease cleavage site (arrowhead) conserved amongst Drosophila Fmi and vertebrate Celsr. (B) The design for Celsr-Activin fusion proteins. A potential pro-region of zebrafish Celsr2 (blue), including the Furin-like cleavage site, is fused to the mature region of mouse Activin A (pink) to generate FmiP-Act (see Materials and methods for details). The potential cleavage site is mutated in alanine. (C-N) Wild-type embryos were injected with 5 pg mouse activin RNA (F-H), 50 pg FmiP-Act RNA (I-K), 100 pg FmiP-Act-RA RNA(L-N) or left uninjected (C-E), and fixed at 50% epiboly to examine expression of the mesoderm or endoderm markers ntl (C,F,I,L), gsc (D,G,J,M) or bon (E,H,K,N). EXPRESSION / LABELING:
|
|
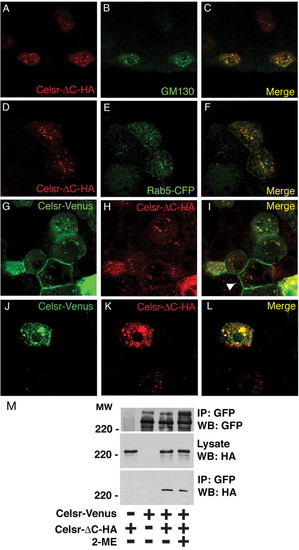
Celsr-ΔC is retained in the golgi and inhibits membrane presentation of Celsr. (A-F) Wild-type embryos were injected with 30 pg Celsr-ΔC-HA DNA alone (A-C) or together with 30 pg Rab5-CFP RNA (D-F) and fixed at 40% epiboly to visualise with an HA antibody (A,D) or either a GM130 antibody for the golgi (B) or a GFP antibody for early endosomes (E). The merge images are shown in C and F. (G-L) Wild-type embryos were co-injected with 30 pg Celsr-Venus DNA and 30 pg Celsr-ΔC-HA DNA, and fixed at 40% epiboly to visualise with a GFP antibody (G,J) or an HA antibody (H,K). The merge images are shown in I and L. When the level of Celsr-ΔC is low, Celsr-Venus is presented at the membrane (shown by arrowhead) (62 cells out of 15 embryos examined but the level of Celsr-ΔC is high, Celsr-Venus is prevented from presenting at the membrane (15 cells out of 15 embryos examined). (M) Non-covalent dimer formation of Celsr. Celsr-Venus and/or Celsr-deltaC-HA were transiently expressed in HEK293 cells. Cell lysates were immunoprecipitated with anti-GFP antibody, and immunoprecipitated proteins were further incubated in Laemmli's buffer with or without 2-mercaptoethanol (2-ME), followed by western blotting with anti-GFP or anti-HA antibody. |
|
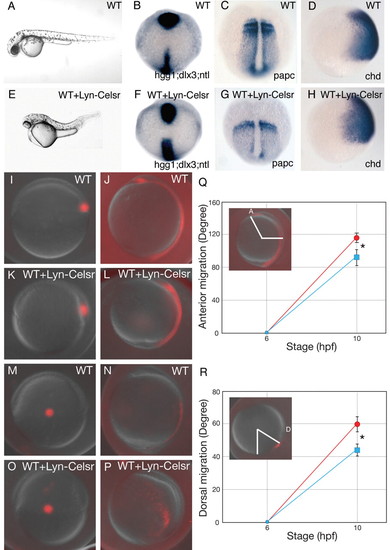
Overexpression of a membrane-targeted intracellular domain of Celsr (Lyn-Celsr) causes a convergence extension defect during zebrafish gastrulation. (A-H) Wild-type (WT) embryos (A-D) or wild-type embryos injected with 100 pg RNA encoding Lyn-Celsr (E-H). (A,E) Lateral views of pharyngula stage living WT (A) and Lyn-Fmi-expressing (E) embryos. The Lyn-Fmi-expressing embryo shows a shorter body axis in this case associated with cyclopia. Dorsal views (B,C,F,G) of tail-bud stage wild-type embryos (B,C) and Lyn-Celsr-expressing embryos (F,G). Lateral views of 80% epiboly WT (D) and Lyn-Celsr-expressing (H) embryos. Anterior is upwards and genes analysed are indicated in the bottom right-hand corner. (I-R) Analysis of axial and lateral mesendermal cells using a photo-conversion strategy. Labelled axial mesendermal cells at shield stage (6 hpf) in control (I) and Lyn-Celsr embryos (K) were analysed at tailbud stage (10 hpf) (J,L, respectively). Labelled lateral mesendermal cells at shield stage (6 hpf) in control (M) and Lyn-Celsr embryos (O) were analysed at tailbud stage (10 hpf) (N,P, respectively). Lateral views (I-P). Quantification of anterior migration of axial cells (Q) and dorsal migration of lateral cells (R). Blue, Lyn-Celsr-expressing embryos; red, WT embryos. Means and s.d. are shown. Asterisks indicate statistically significant differences (P<0.05; Student's t-test). Note that Lyn-Celsr-expressing embryos show both convergence and extension defects. EXPRESSION / LABELING:
|
|
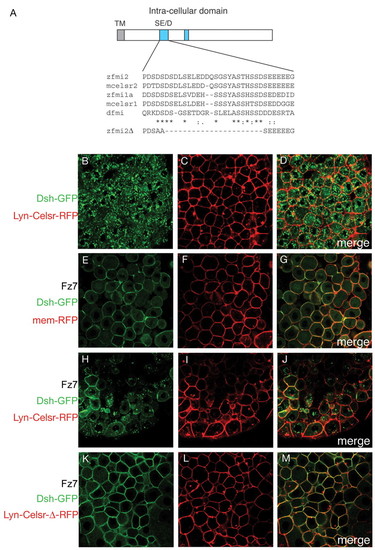
Lyn-Celsr inhibits Frizzled-induced membrane localisation of Dishevelled through a highly conserved SE/D domain. (A) Schematic illustration of the intracellular region of Fmi/Celsr. Two domains are conserved among vertebrates as highlighted in blue. Only one of these, the SD/E domain is conserved between vertebrates and Drosophila. The partial sequence of a construct lacking this domain is shown. (B-M) Wild-type embryos injected with 150 pg Dsh-GFP RNA in the absence (B-D) or presence (E-M) of 100 pg Fz7 RNA, together with 100 pg membrane-RFP (E-G), Lyn-Celsr-RFP (B-D,H-J) or Lyn-Celsr-Δ-RFP (K-M), as indicated and analysed at 40% epiboly by confocal microscopy. Scanning for GFP and RFP was carried out simultaneously and merged (D,G,J,M). Fz7-mediated membrane localisation of Dsh is inhibited by Lyn-Celsr (H,J) but not by Lyn-Celsr-Δ (K,M), which lacks the SD/E domain conserved between vertebrates and Drosophila (A). |
|
Differential cell cohesive properties of wild-type cells compared with cells from celsr mutant/morphants and cells from embryos expressing Celsr-ΔC. Wild-type and MZord embryos were injected with RNA/morpholinos together with fluorescein-dextran (FDX, green) or rhodamine-dextran (RDX, red), as indicated. (A) 30-40% epiboly embryos were dissociated and dissociated cells from different populations were mixed in a minimal volume of a hanging drop and kept overnight to analyse aggregates by confocal microscopy. (B) Cells from wild-type embryos (green) and wild-type embryos (red). (C) Cells from MZord embryos injected with 0.4 pmoles each of celsr1a and celsr1b morpholinos (green) and wild-type embryos (red). (D) Cells from wild-type embryos injected with 300 pg Celsr-ΔC-HA RNA (green) and wild-type embryos (red). (E) Cells from wild-type embryos injected with 100 pg Lyn-Celsr RNA (green) and wild-type embryos (red). Celsr mutant/morphant cells (C) or Celsr-ΔC-expressing cells (D) are strongly segregated from wild-type cells, whereas Lyn-Celsr-expressing cells are only weakly segregated from wild-type cells (E). PHENOTYPE:
|
|
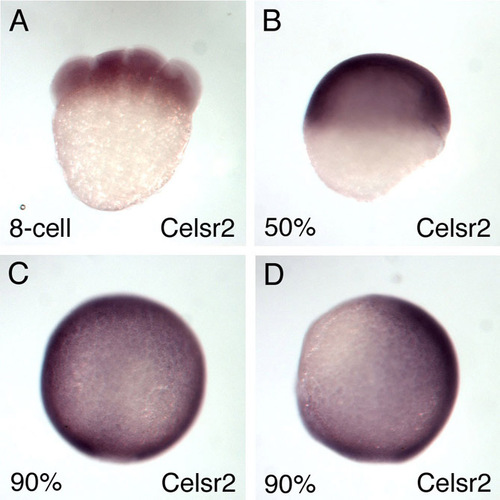
Expression of celsr2 gene during gastrulation in zebrafish. celsr2 is expressed maternally (A) and is expressed ubiquitously at 50% epiboly (B) (lateral view, dorsal to the right) and at 90% epiboly dorsal view (C) and lateral view, dorsal towards the right (D). |
|
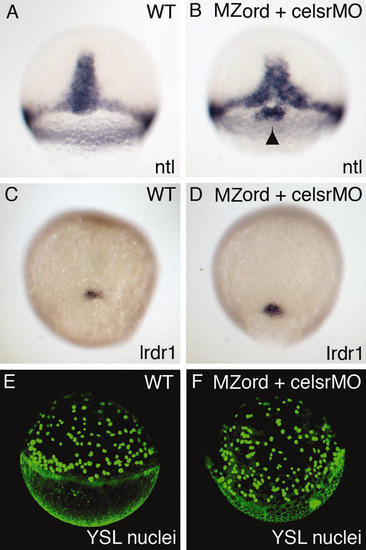
celsr mutant/morphants do not either exhibit defective formation of dorsal forerunner cells or defective movement of the YSL nuclei. (A-F) Wild-type embryos (A,C,E) or MZord embryos injected with 0.4 pmoles each of celsr1a and celsr1b MOs (B,D,F) were visualised for dorsal forerunner cells with ntl probe at 70% epiboly (indicated by an arrowhead in B) or with lrdr1 probe at 90% epiboly (C,D). The embryos were injected with SYTOX green at sphere stage, and then visualised for YSL nuclei at 50% epiboly (E,F). |
|
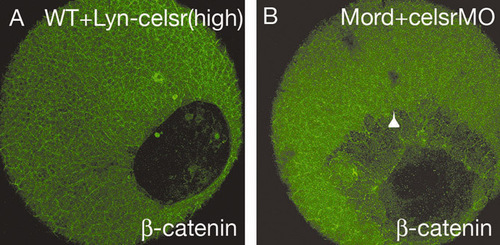
Maternal Celsr2 is required for epiboly, whereas Lyn-Celsr expression does not result in epiboly defects in wild-type embryo. (A) Wild-type embryos were injected with 300 pg RNA encoding Lyn-Celsr. (B) Embryos from crossing between MZord female with TL wild-type male were injected with 0.4 pmoles each of celsr1a and celsr1b morpholinos. The embryos were fixed at 90% epiboly for β-catenin antibody staining. An arrowhead indicates the leading edge of deep cells. Note that embryos expressing Lyn-Celsr show no epiboly phenotype (n=33). |
|
Celsr-ΔC causes C&E and epiboly defects, and injection of a low dose of stbmMO, dvl2MO or Lyn-Celsr causes C&E but not epiboly defects in MZord embryos. MZord embryos (A,D) or MZord embryos injected with 0.4 pmoles stbmMO (B,E), 0.75 pmoles dvl2MO (C,F) or 20 pg Lyn-Celsr RNA (I,L). Wild-type embryos were injected with 300 pg Celsr-ΔC-HA RNA (H,K) or left uninjected (G,J). The embryos were fixed at tail-bud and subjected for in situ hybridisation with hgg1 for the prechordal plate, ntl for the notochord and dlx3 for the anterior edge of the neural plate. The results of C&E phenotypes are summerised in Table S1. Note that injection of 300 pg Celsr-ΔC-HA RNA causes C&E defects with posteriorly misplaced prechordal plate as well as epiboly defects shown by the shifted germ ring expression of ntl (indicated by an arrowhead). |
|
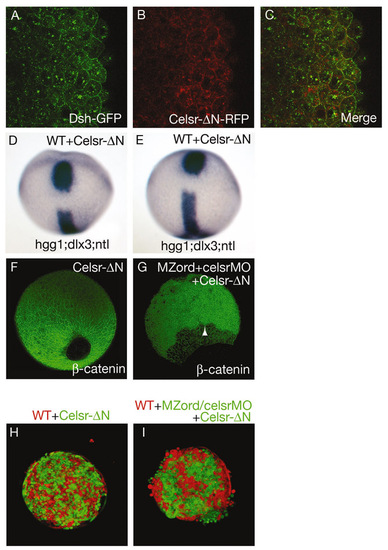
Characterisation of Celsr-ΔN. (A-C) Wild-type embryos were injected with 150 pg Dsh-GFP together with 100 pg Celsr-ΔN-RFP RNA. Scanning for GFP (A) and RFP (B) was carried out simultaneously and merged (C). Note that Celsr-ΔN is capable of recruiting Dsh without exogenous Fz, suggesting activation of the Wnt/PCP pathway. (D,E) Celsr-ΔN causes CE defects. Wild-type embryos were injected with 100 pg Celsr-ΔN-Venus RNA, and fixed at tail-bud for staining with hgg1 for the prechordal plate, ntl for the notochord and dlx3 for the anterior edge of the neural plate (see Table S1 in the supplementary material). (F,G) Effects of Celsr-ΔN on epiboly. Injection of a high dose (300 pg) Celsr-ΔN-Venus RNA does not lead to epiboly defects (F), whereas expression of 100 pg Celsr-ΔN-Venus RNA fails to rescue the celsr mutant/morphant phenotype (G; see Table S2 in the supplementary material). An arrowhead indicates the leading edge of deep cells. (H,I) Hanging drop assays. Cells from wild-type embryos with cells from embryos expressing 300 pg Celsr-ΔN RNA, being intermingled well (H) (n=11). Expression of 50 or 100 pg Celsr-ΔN-Venus RNA fails to restore altered cell cohesive property of celsr mutant/morphant cells (I) when compared with Fig 7C. Note that there is a strong correlation of epiboly defects with altered cell cohesive properties. |