- Title
-
Nodal signaling is required for closure of the anterior neural tube in zebrafish
- Authors
- Aquilina-Beck, A., Ilagan, K., Liu, Q., and Liang, J.O.
- Source
- Full text @ BMC Dev. Biol.
|
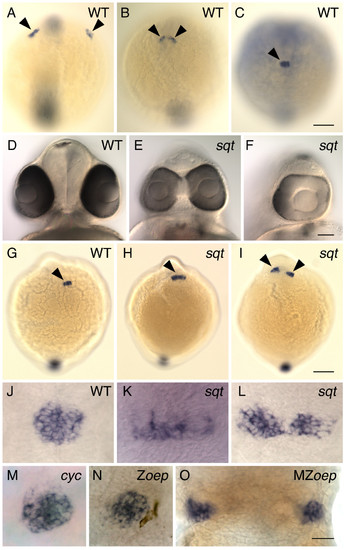
Pineal precursors fail to reach the midline of the forebrain in squint mutants. (A-C) WT embryos were processed for whole mount in situ hybridization with an antisense probe for the gene flh, which is expressed in the pineal precursors (arrowheads). Images are dorsal views of the entire embryo, anterior to the top. (A) At the 2–3 somite stage, pineal precursors are located in two widely spaced lateral domains. (B) By the 5–6 somite stage, these domains have moved towards the dorsal midline of the forebrain. (C) By the 7–8 somite stage, a single, round-shaped pineal anlage has formed. (D-F) At 2 dpf, sqt mutant embryos from the same clutch have a wide range of eye phenotypes. Frontal views of live embryos with dorsal to the top. (D) The eyes of WT embryos and some sqt mutants (not shown) are completely separated from one another. (E) Other sqt mutants have partially fused eyes that form two lenses or (F) a single eye with one lens. (G-L) Embryos at the (G-I) 7–8 somite stage and (J-L) 24 hpf were processed for whole mount in situ hybridization with a probe for the pineal gene (G-I) flh or (J-O) otx5, dorsal views, anterior to the top. (G,J) In WT siblings, the pineal precursors (arrowhead) have converged to form a round pineal anlage. In sqt mutants the pineal precursors (arrowheads) form a domain that is (H,K) elongated or (I,L) divided in two. The pineal anlagen of the (M) cyc mutant and the (N) Zoep mutant have a round shape that is similar to that of WT fish, while the pineal precursors of the (O) MZoep mutant are divided in two domains. All images are dorsal views with anterior to the top. Scale bars: 100 μm (A-C, G-I), 70 μm (D-F), 30 μm (J-O). EXPRESSION / LABELING:
PHENOTYPE:
|
|
Elongated and divided phenotypes in squint mutants persist through the first three days of development. The morphology of the pineal was followed over time in individual sqt mutants and WT siblings carrying the flh:eGFP transgene, which expresses GFP throughout the pineal anlage during embryogenesis [21]. All sqt mutants used in this experiment had cyclopic eyes. (A-D) Composite bright field and fluorescent images at 2 dpf showing fluorescence in the pineal anlage of embryos having (A-B) normal, round-shaped pineal anlagen, and (C) elongated and (D) divided pineal anlagen. (E-H") Each row shows the pineal of an individual embryo over three days of development as assayed by fluorescence microscopy on a compound microscope. Note the similarity in the morphology of the pineal in (E-E") the WT embryo and (F-F") one of the sqt mutants. In contrast, the sqt mutants in G-G" and H-H" maintain their abnormal elongated and divided morphologies throughout the experiment. All images are dorsal views with anterior to top. Scale bars: 60 μm (A-D), 30 μm (E-H"). EXPRESSION / LABELING:
PHENOTYPE:
|
|
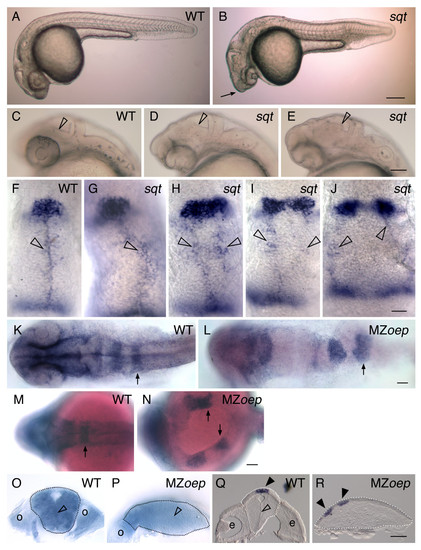
Correlation between an expanded or divided pineal and an open neural tube. (A-E) Lateral views of live embryos at 1 dpf, anterior to the left. (A) While the head of the WT embryo is smooth and rounded, (B) the head of the sqt embryo is pointed (arrow). (C-E) Higher magnification of the anterior embryo reveals variability in the brain morphology of sqt mutants. In (C) WT embryos and (D) some sqt mutants, the border between the tectum and tegmentum (open arrowheads) appears as a smooth, straight line. (E) However, in some sqt mutants the border appears to be abnormally shaped or indistinct (open arrowhead), suggesting that the morphology of tectum or tegmentum is perturbed. (F-J) Embryos were fixed at 1 dpf, and processed for in situ hybridization with antisense probes for the pineal gene otx5 and the dorsal neural tube gene wnt1. In (F) WT and (G) sqt embryos with a single, round pineal anlage, the wnt1 expressing cells (open arrowheads) form a single domain along the dorsal neural tube. In contrast, sqt embryos with an (H) elongated or (I-J) divided pineal anlage have two parallel lines of wnt1 expressing cells. (K-P) Embryos were fixed at 1 dpf, processed for in situ hybridization with an antisense probe for epha4a, and then either (K-N) imaged in dorsal view, anterior to the left or (O, P) cut through epha4a-expressing rhombomere 5 to bisect the embryo into anterior and posterior halves. The locations of the otic vesicles (o), rhombomere 5 (arrows), and midline (open arrowhead) are indicated. A potential region of midline is marked by the open arrowhead in P. (Q, R) 14 μM frozen cross sections through the diencephalon of 1 dpf (Q) WT or (R) MZoep embryos stained for otx5 expression. The midline of the brain (open arrowhead), and pineal precursors (closed arrowheads) are indicated. Dotted lines outline the neural tubes in panels O-R. Scale bars: 200 μm (A,B), 100 μm (C-E), 30 μm (F-J), 50 μm (K-R). |
|
The expanded and divided pineal phenotype is present in n-cad mutants and morphants. (A-C) Homozygous pac mutants were fixed at 30 hpf, and then processed for in situ hybridization with an antisense probe for otx5. Dorsal views with anterior to the top. pac mutants could have a (A) normal, round-shaped pineal morphology, (B) an elongated pineal anlage or (C) a divided pineal. (D-G) Embryos were injected at the one to four cell stage, fixed at 74 hpf, and then assayed for expression of otx5. Frontal views with dorsal to the top. The presumptive pineal organ (arrowheads) forms (D) a single domain in control embryos and a (E-G) divided pineal in embryos depleted in N-cad through morpholino (MO) injections. Scale bars: 30 μm (A-C), 100 μm (D-G). EXPRESSION / LABELING:
PHENOTYPE:
|
|
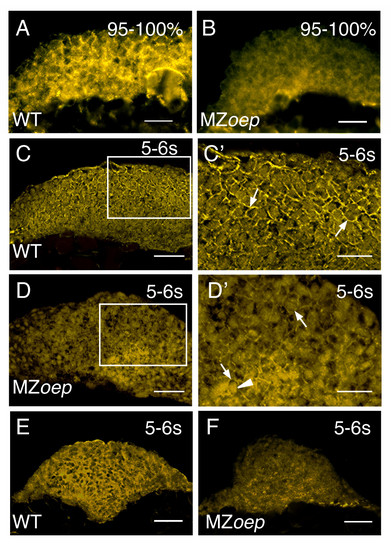
N-cad expression in the anterior neural keel is altered in MZoep mutants. (A-F) N-cad immunostaining of tissue sections from WT and MZoep embryos. (A, B) Sagittal sections though the anterior neural keel with anterior to the left and dorsal to the top. (C-D') Parasagittal sections with anterior to the left and dorsal to the top. Panels C' and D' are higher magnifications of the boxed regions in panels C and D, respectively. Arrows point to N-cad labeling in the cell membrane, while the arrowhead indicates labeling in the cytoplasm. (E, F) Transverse sections through the anterior neural tube, dorsal to the top. n e 30 embryos for each sample. Embryos are at 95–100% epiboly (95–100%) or the 5–6 somite stage (5–6 s). Scale bars: 40 μm (C, D, E, F), 20 μm (A, B, C', D'). EXPRESSION / LABELING:
|
|
The ordered structure of the neural tube is disrupted in MZoep embryos. (A-H) Embryos were injected at the one cell stage with mGFP mRNA and imaged (A-F) live at 95% epiboly (95%), the 4–5 somite stage (4–5 s), the 10 somite stage (10 s), or (G, H) fixed at ~24 hpf. (A-F) Cross sections through the anterior developing neural plate, with presumptive eyes (e) indicated in panel B, the outer boundary of the neural tube indicated by a dotted line in panels B and C, and the midline indicated by the open arrowheads in C. (G, H) Horizontal sections through the midbrain and hindbrain of ~24 hpf embryos, with the midline of the brain (open arrowheads) and the otic vesicles (o) indicated in panel G. (I-J′) Embryos were injected at the one cell stage with DNA encoding mGFP, raised to ~24 hpf, and imaged live in high magnification horizontal sections. Open arrowheads indicate elongated cells and the closed arrowheads indicate round cells. All images are confocal optical sections. Scale bars: 40 μm (A-H), 80 μm (I-J′). |
|
Injection of n-cad mRNA into MZoep embryos. Embryos were injected at the one cell stage with n-cad mRNA, raised to approximately 24 hpf, and fixed and processed for whole mount in situ hybridization with a probe for otx5. (A) WT and (B, C) some N-cad overexpressing MZoep embryos have a round shaped pineal morphology indicative of a closed pineal organ. (D, E) Other N-cad overexpressing MZoep fish have an elongated or divided pineal organ, demonstrating that their NTD has not been corrected. All images are dorsal views with anterior to the top. The background in Panel C has a pink cast because the embryo was too fragile to remove from the yolk. The midline of the brain is apparent in panels A and B, and is indicated by a black line. Scale bar: 40 μm (A-E). |
|
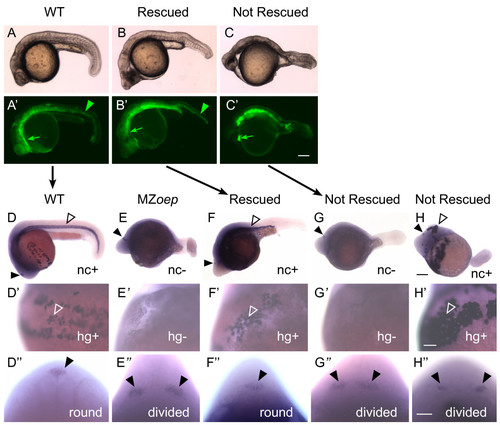
Taram-A* expression results in recovery of mesendoderm and neural tube closure in MZoep mutants. Embryos were co-injected with Taram-A* and mGFP mRNA into one blastomere at the 16 cell stage, raised until 24 hpf, and imaged (A-C′) live by (A-C) bright field and (A′-C′) fluorescent microscopy, and then (D-H") fixed and processed for whole mount in situ hybridization with otx5 to mark the pineal, ctsl1b to mark the hatching glands (hg), and col2a1a to mark the notochord (nc). Panels with the same letter are different views of the same embryo. In addition, the embryos shown in the A, B, and C rows are the same embryos as in the D, F, and G rows, as indicated by the black arrows. (A′-C′) Green arrows indicate fluorescence in the anterior mesendoderm, green arrowheads fluorescence in the notochord. (D-H) Closed arrowheads indicate the pineal organ, and open arrowheads the notochord. Whether an embryo is positive (+) or negative (-) for notochord staining is indicated. (D′-H′) Open arrowheads point to hatching gland cells, and whether the embryo is positive or negative for these cells is indicated as in D-H′. (D"-H") Closed arrowheads point to the regions of pineal precursors, and the morphology of the pineal anlage is noted. (A-H′) Sagittal views with anterior to the left. (D"-H") Dorsal views of the back of the head. Scale bars: 100 μm (A-H), 50 μm (D′-H"). EXPRESSION / LABELING:
|
|
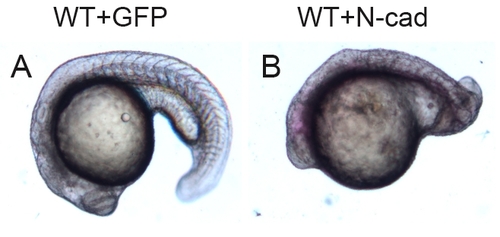
Injection of N-cad mRNA disrupts development of WT embryos. Embryos were injected with 75 pg of mGFP or N-cad mRNA and raised to approximately 24 hpf. Lateral views with anterior to the left. Note that the N-cad overexpressing embryo in (B) has a smaller head and shortened body axis compared to the control injected embryo in (A). |