- Title
-
Conserved sensory-neurosecretory cell types in annelid and fish forebrain: insights into hypothalamus evolution
- Authors
- Tessmar-Raible, K., Raible, F., Christodoulou, F., Guy, K., Rembold, M., Hausen, H., and Arendt, D.
- Source
- Full text @ Cell
|
Conserved Regionalization of the Zebrafish and Platynereis Forebrain (A, E, and F) Dorsal and (B) lateral views of zebrafish embryos hybridized with the indicated riboprobes; stages as indicated. (A, E, and F) Anterior to the top and (B) to the left, (B) eyes removed. (C, G, and H) Apical views of Platynereis embryos hybridized with the indicated riboprobes. (D) SEM picture of a Platynereis larva. Pro, prostomium; per, peristomium; met, metastomium; mo, mouth. (I and J) Expression synopsis of zebrafish nk2.1a, pax6.1, and rx3 (I) and of the polychaete orthologs (J). Yellow, anterior neuroectoderm; med, medial; lat, lateral forebrain. |
|
Vasotocinergic Cells in the Developing Medial Neurosecretory Forebrain. (A–C) Differentiating neurosecretory cells in the Platynereis medial forebrain. Circles demarcate the location of the studied vasotocinergic and RFamidergic cells in comparison to axonal scaffold and position of sections shown in (D)–(F). (D–F) Transmission electromicrographs of the Platynereis medial forebrain plexus; black arrowheads, dense core vesicles (DCV); white arrowheads, synaptic vesicles (SV); scales as indicated. (D) Synapse containing DCV and SV. (E) DCV release, indicating larval neurosecretory activity. (F) Primary body cavity (black arrows) reaching into the plexus. (G) Scheme of (F). (H) Scheme of Platynereis-Vasotocin-Neurophysin (Pdu-Vtn), based on Figure S2. Percentages: identity of Mus ArgVasopressin-Neurophysin to Pdu-Vtn (in brackets: to zebrafish Vtn). Arrow demarcates the predicted cleavage site behind the nonapeptide. Different colors indicate the degree of conservation to other lophotrochozoans: complete (red), partial (yellow), none (black). (I–L) Pdu-vtn and Pdu-c-opsin expression localizes to the same landmarks (axons and large cilia of extraocular PRCs). (Note that these cells do not appear to bear the prominent cilia themselves.) Coexpression could not be assayed directly due to technical limitations (Tessmar-Raible et al., 2005). (M and N) Zebrafish vtn and tmt-opsin are coexpressed (arrows) by cells adjacent to the postoptic commissure/optic chiasm (blue arrows). (O) Summary schemes of zebrafish and Platynereis larval brains, indicating the position of vasotocinergic cells (yellow) with respect to the axon tracts (white) and nk2.1 expression (red). Anterior to the top. Blue arrows indicate positions of optic commissures (POC/OC). Stages, riboprobes, and antibodies as indicated. Asterisks demarcate the position of the neurosecretory forebrain plexus; yellow arrowheads, large cilia of deep brain photoreceptor cells; white arrows (J and L), projections of vtn+ cells into the plexus; blue arrows, entry of optic fibers into the axonal scaffolds of both Platynereis and zebrafish. (A–C and I–L) Apical view, ventral down; (M–O) ventral view, anterior to the top. AC, anterior commissure; POC/OC, postoptic commissure/optic chiasm; PC, preoral commissure. Depth of confocal reconstructions: (A) 31 μm, (B) 35 μm, (C) 30 μm, (J) 15 μm, (L) 18 μm. |
|
RFamidergic Neurons in Platynereis and Zebrafish Medial Forebrains. (A–C) Immunostainings/in situ hybridizations of RFamidergic, otp+ cells in the apical plexus of Platynereis embryos. (A and B) Same embryo, different confocal projections, showing the RFamidergic plexus (B) below the contributing cells ([A], same level as [C]). (D–F) Transmission electromicrographs and derived reconstruction (G). (D) TEM view of a medial flask-shaped cell. Inset: enlargement of the dendrite rich in microtubules (black arrowheads). (E) Two cilia protruding from a medial flask-shaped cell to the subcuticular space. Inset: cilium in cross-section. (F) Cilium of the branching type; black arrowheads, ramifications extending underneath the cuticle. (H) zebrafish RFamidergic cells and (I and J) Dr-farp1 transcript in relation to Dr-otp1 (red in [I]) and the axon scaffold (green in [J]). (K) Forebrain CSF-contacting neuron (according to data from Leonhardt, 1980). AC, anterior commissure; POC/ OC, postoptic commissure/optic chiasm. Views: (A–F) apical, (A–C) ventral to the bottom; (G) ventroapical; (H and I) ventral, anterior to the top; (J) lateral, anterior left. White arrows: position of large multiciliated crescent cell as landmark for orientation, yellow arrowheads: microtubule-rich dendrites bearing cilia. Depth of confocal reconstructions: (A and C) 7 μm, (B) 3 μm. |
|
miR-7 Demarcates Specific Groups of Cells in the Medial Forebrain of Platynereis and Zebrafish. Expression of miR-7 in relation to the axonal scaffold (green) in (A–C) Platynereis and (D–G) zebrafish. (A–C) Apical views, ventral to the bottom. White arrows: position of large multiciliated crescent cell as landmark for orientation; yellow arrowheads: microtubule-rich dendrite; asterisks: position of neurosecretory forebrain plexus. (D, F, and G) Lateral views, anterior to the left, (E) ventral view, anterior: top. Yellow and white circles outlines the same groups of cells in Figures 3M, 4J, and 5E–5G. AC, anterior commissure; POC/OC, postoptic commissure/optic chiasm. |
|
Regulatory Signature of vtn+ and RFamidergic Cells and Dependence of Dr-vtn on Dr-rx3. Expression of indicated genes in (A–E) Platynereis and (G–O) zebrafish. (B and C) Expression in relation to the axonal scaffold (green), compare to Figures 3A–3C, 3J, 3L, and 5B for colocalization with Pdu-vtn, Pdu-c-opsin, miR-7. Yellow arrowheads: large cilia of deep brain ciliary photoreceptor cells. (F) Scheme of (D) and (E). The overlap of all three transcription factors (white) includes the medial vtn+ cells (white arrows in [D] and [E]). Overlap of nk2.1 and otp (yellow) in the medial RFamidergic cells (white arrowhead in [D]). (G–L) White circle demarcates same group of cells. Yellow arrow: Dr-farp1+ cells. (N and O) Absence of Dr-vtn expression in wild-type (WT) versus chks399 (chokh) siblings. (P) Summary of regulatory analyses, all assayed at 27–28 hpf. Morpholino-assisted knockdown of rx3 leads to dose-dependent reduction/absence of vtn expression at 27–28 hpf compared to controls (ctrl). Loss of vtn expression in early differentiating cells in two homozygous rx3 mutant alleles (chk s399 and chk ne2611). Below each bar: numbers of investigated specimens and volume of injected morpholino solution/genotype of fish as judged by presence/absence of eyes. Views: (A–F) apical, ventral to the bottom, (G–O) lateral, anterior left. |
|
Early born vtn cells form in the small co-expression area of rx3 and otp1. Lateral views of embryos hybridized with the indicated riboprobes; anterior left. Arrows in C,D indicate the initiation of the early born vtn at the small overlap of rx3 and otp1. (Note that this overlap also includes nk2.1a/b, data not shown). EXPRESSION / LABELING:
|
|
Development and differentiation of the forebrain is not abolished in chks399 mutant fish. Gene expression in chks399 (chokh) fish vs. sibling (wt). Stages and probes as indicated. Views: (A,B,E,F,I-P) lateral, anterior to the left. (C,D,G,H,Q-T) ventral, anterior to the left. Note that the early-born vtn cells that are not initiated in homozygous chk mutant fish are also absent at later stages (black arrows in A,B) with 100% penetrance. (compare to Fig. 6). EXPRESSION / LABELING:
|
|
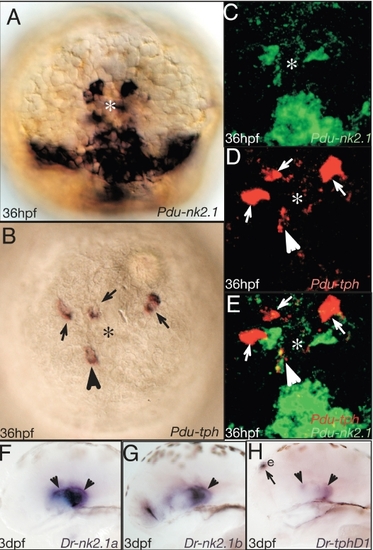
Tryptophane hydroxylase localize to the nk2.1+ region. (A-E) Apical views on the Platynereis forebrain, ventral to the bottom. E is a merged views of C and D. Arrowhead points at Platynereis tryptophan hydroxylase (Pdu-tph) positive (i.e. serotonergic) cells. (F-H) Correlation of serotonergic and nk2.1 cells in the fish hypothalamus. Arrowheads point at tph cell co-expressing nk2.1. Arrows point at cells not co-expressing nk2.1. Riboprobes and stages as indicated. e: epiphysis. EXPRESSION / LABELING:
|
Reprinted from Cell, 129(7), Tessmar-Raible, K., Raible, F., Christodoulou, F., Guy, K., Rembold, M., Hausen, H., and Arendt, D., Conserved sensory-neurosecretory cell types in annelid and fish forebrain: insights into hypothalamus evolution, 1389-1400, Copyright (2007) with permission from Elsevier. Full text @ Cell