- Title
-
meis1 regulates cyclin D1 and c-myc expression, and controls the proliferation of the multipotent cells in the early developing zebrafish eye
- Authors
- Bessa, J., Tavares, M.J., Santos, J., Kikuta, H., Laplante, M., Becker, T.S., Gómez-Skarmeta, J.L., and Casares, F.
- Source
- Full text @ Development
|
meis1 retracts accompanying the ath5 wave and becomes restricted to the CMZ. (A-D) meis1 and (E-H) ath5 expression analyzed by single in situ hybridization. Developmental stages are indicated as hours post-fertilization (hpf) at 28.5°C. Lateral views of whole-mount (A,E) or dissected (B-D,F-H) eyes, with dorsal up and anterior to the left. The front of the ath5 domain is marked by black arrowheads. The red arrowhead in D points to meis1 expression in the prospective ciliary margin. (I-M) Transverse 40 μm vibratome sections. (I,J) Dorsal is up. meis1 is weakly expressed in the lens ectoderm before its thickening (I), but no signal is detected once the lens placode is formed (J). (K-M) Dorsal is to the left. meis1 and ath5 expression domains are complementary as shown by double in situ hybridization (K,L). Approximate limits of the ath5 signal are indicated by the black arrowheads. (M) At 42hpf, meis1 expression is detected by in situ hybridization in the ciliary margin (red arrowheads) and in the postmitotic ganglion cells (black arrowhead). L, lens. EXPRESSION / LABELING:
|
|
meis1 is required for the growth of the eye primordium. (A,B) Lateral views of representative 72hpf control-MO (A) and meis1-MO (B) -injected fish. meis1 morphants are microphthalmic. (C,D) Confocal images of dissected eyes stained for propidium iodide (nuclei), rhodamine-phalloidin (filamentous actin) and Islet1, which labels GCL nuclei and some in the INL. The reduced eyes from meis1 morphants show apparently normal retina lamination (D,D′), but fewer cells than control eyes (C,C'). Area (E) and estimated volume (F) of control-MO and meis1-MO-injected embryos at 72hpf. meis1- morphant embryos show a significant (P<0.001) reduction in eye area and volume (45% and 60%, respectively). n=20 for each condition. PHENOTYPE:
|
|
meis1 is required for the G1-S transition and the expression of the G1-S regulators cyclin D1 and c-myc. (A-D) Histograms displaying DNA content (cell cycle profile) of cells from dissected 19hpf eyes of meis1-MO treated embryos relative to (A) controls (Crtl-MO), (B) meis1-MO+GFP-meis1 mRNA, (C) meis1-MO+cyclin D1 mRNA, and (D) meis1-MO+c-myc mRNA injected embryos. Number of replicates (n) and P values are indicated. meis1 knockdown induces a delay in G1, which is partially rescued by co-injection of GFP-meis1 (B), cyclin D1 (C) and c-myc (D) mRNAs. (E) Average percentages are shown for G1, S and G2/M DNA content. The cell-cycle profiles of control morphants and of uninjected, wild-type embryos are indistinguishable (not shown). (F-I) In situ analysis of cyclin D1 and c-myc transcription in control (F,H) and meis1 morphants (G,I) at 19hpf. meis1 morphants show a dramatic reduction in cyclin D1 and c-myc in the eye (red arrowheads). cyclin D1 and c-myc are still detected in other body regions (black arrowheads). Hybridization and reaction development were performed strictly in parallel. Representative embryos are shown. EXPRESSION / LABELING:
|
|
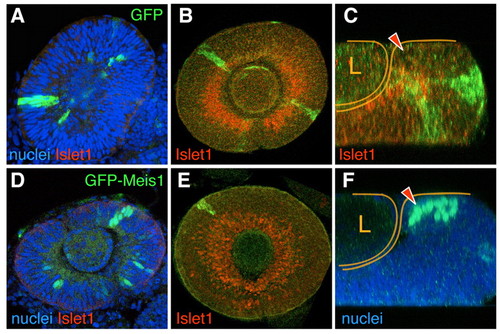
Clonal overerexpression of meis1 in the developing eye prevents differentiation and results in cell sorting. Single optical sections from confocal z-stacks of GFP (A-C) or GFP-Meis1 (D-F) expressing clones induced genetically in developing eyes. GFP-meis1 signal is nuclear. At 24-30hpf, both clone types frequently span the whole width of the neuroepithelium (A,D). Confocal optical sections through the central retina (B,E) and z-sections (C,F) of 48hpf eyes. At this stage, GFP clones comprise both Islet1-expressing and non-expressing cells (B,C). By contrast, same-stage GFP-Meis1 clones in the central retina do not contain Islet1-positive cells (E). GFP-Meis1 clones are often located in the CMZ (F*ge, 9. The arrowheads (C,F) point to the CMZ, and the retina and the lens (L) are outlined. |
|
Morpholino (MO) sequences, targets and specificity assay in Xenopus. (A) Alignment of Meis sequences around the ATG (underlined). Morpholino (targeted) sequences are shadowed in gray. The control mopholino used in this paper (Ctrl-MO) was originally designed to target the ATG region of Xenopus tropicalis Olig2. It does not target any ATG in the zebrafish genome. (B) Mismatches between meis1, meis2.2 and meis3 morpholinos and the ATG regions of non-target Meis genes. All but one have five or more mismatches, which should prevent cross-targeting. The exception is meis2.2-MO, which might cross-target meis4. This fact did not interfere in our analysis since meis4 is not expressed in the developing eye. meis3-MO, which has nine and seven mismatches with meis1 and meis2.2, respectively, is included here as it also served as control MO in some experiments. (C-F) Specificity assay in Xenopus laevis embryos. meis1 and meis2.2 mRNAs, carrying a C-terminal Myc tag (meis1-Myc and meis2.2-Myc) (10 ng), were co-injected with either meis1 or meis2.2 MOs (1 ng) in two-celled Xenopus embryos. After injection, embryos (n>50 per experiment) were allowed to develop for 24 hours at 18°C, fixed and immunostained to detect the Myc tag (e.g. Tena et al., 2007). Effective targeting results in the block of mRNA translation and no production of the Myc-tagged protein. Each MO blocks the translation of only its specific mRNA (C,F; absence of nuclear Myc staining). Accordingly, meis1-MO is unable to block meis2.2-Myc translation, as meis2.2-MO is incapable of blocking that of meis1-Myc (B,E; presence of nuclear Myc staining, brown). |
|
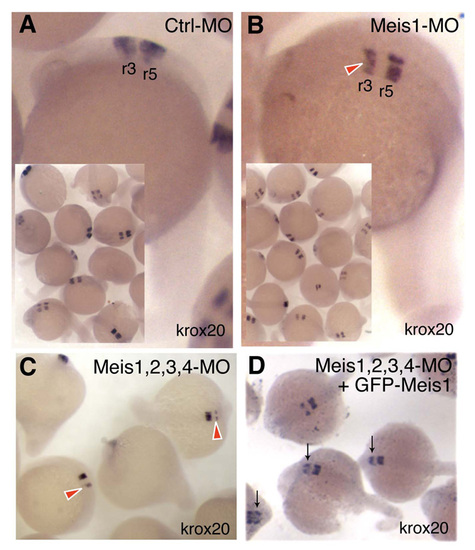
meis1-MO injection reduces rhombomeres-3 krox20 expression specifically. (A) Ctrl-MO has no effect on krox20 expression in rhombomeres 3 (r3) or 5 (r5). (B) meis1-MO embryos show a specific reduction of r3 krox20 expression (red arrowhead). (C) Injection of a meis1, 2.2, 3 and 4 MO cocktail results in an even stronger inhibition of r3 krox20 expression (red arrowheads). (D) This effect is partially rescued by injection of GFP-meis1 mRNA (meis1GFP) (black arrows). GFP-meis1 is insensitive to meis1-MO, since the N-terminal tagging of meis1 with GFP destroys the meis1-MO target sequence in the GFP-meis1 mRNA. These results phenocopy the reported effect on r3 krox20 expression caused by injection of a dominant-negative Meis form (Waskiewicz et al., 2001) and thus confirm the biological specificity of the Meis mopholinos used in this study. EXPRESSION / LABELING:
|
|
Expression of meis1, meis2.2 and meis2.1 during eye development. (A-H) Dorsal views of flat-mounted embryos. meis1 and meis2.2 are expressed uniformly throughout the optic vesicle (A,E) and early-stage optic cup (B,F). After this developmental point, meis2.2 expression fades (G,H), while meis1 becomes progressively restricted to the peripheral retina (C,D). Inset in B is a flat mount of a 16-hpf meis1-YFP showing uniform YFP signal in the optic vesicle, which recapitulates meis1 expression at this stage. (I-K,M-O) Lateral views of whole-mount embryos. meis2.1 and meis2.2 are not expressed in the eye (open arrowheads) at 24 hpf (I,M). Both genes begin to be expressed in a central patch of the neural retina at about 33-36 hpf (J,N) that becomes a thin rim of cells by 48-52 hpf (K,O). (L,P) 40-μm vibratome sections of gelatin-embedded embryos show that, at 48-52 hpf, meis2.1 and meis2.2 are expressed in postmitotic ganglion cells. (Q-S) 15-μm cryostat sections of eyes from 4-dpf fish, hybridized with probes against (Q) meis1, (R) meis2.1 and (S) meis2.2. At this stage, all three genes are expressed in the GCL and in the most central region of the INL - likely amacrine cells - but at different relative levels. Also at this stage, only meis2.2 is expressed at significant levels in the ciliary margin zone (CMZ). Arrowheads indicate expression detected by in situ hybridization. hpf and dpf, hours and days post-fertilization, respectively, at 28.5°C. The meis1-YFP enhancer trap is an insertion of a YFP-retroviral vector (Laplante et al., 2006) located 1,707 bp downstream of the last exon of meis1 (chr13) and inserted in the same transcriptional direction. EXPRESSION / LABELING:
|
|
Meis/Hth genes are not essential for pax6/ey expression. (A,B) pax6b, detected at 20 hpf by in situ hybridization, is expressed at similar levels in both control-MO (A) and meis1-MO (B) injected embryos, despite the already noticeable reduction of the eye primordium in meis1-morphants. We have noticed, though, a consistent reduction of pax6b expression in the lens of meis1 morphants. (C) RT-PCR of two independent experiments (#1 and #2) for pax6b and actin, as internal control. Total RNA, from dissected eyes or remaining bodies of 19-hpf injected embryos (control-MO and meis1-MO), was isolated using an RNeasy Kit (Qiagen) and cDNA prepared with standard methods. PCR was performed with specific primers for pax6b, forward 5′-GATTTTGCAGGTGTCGAATG-3′ and reverse 5′-TTCACCACCGTTGTCCTGT-3&prime& β-actin primers as in (Duffy et al., 2005). PCR protocol used: denaturation of 95°C for 2 minutes, followed by 28 cycles of 95°C for 1 minutes, 60°C for 30 seconds, and 72°C for 1 minute, and a final elongation of 72°C for 5 minutes. (D) The image processing and pixel intensity values of ethidium bromide-stained agarose gels were obtained with Typhoon 9410 Variable Mode Imager. Results from two independent experiments (each performed in duplicate), are expressed as mean±STDEVP (standard deviation using the entire data population as argument). (E,F) Drosophila late third larval stage eye disc containing clones of cells mutant for a strong hth hypomorphic allele, hthP2. The clones are marked by the absence of CD2 (clones are outlined in F). ey expression remains unaltered in hth mutant cells. Hth clones were induced between 48 and 72 hours after egg laying by a 20-minute 37°C heat shock on larvae resulting from the cross of FRT82B hthP2/TM2 males to yw hsFLP 122; FTR82B hsCD2 y+ M/TM2 females (e.g. Pichaud and Casares, 2000 20-minute 37°C heat shock on larvae resulting from the cross of FRT82B hthP2/<29. Expression of CD2 was induced by a 30-minute heat shock at 37°C, 30 minutes prior to dissecting wandering third instar larvae. Mouse anti-CD2 is from Serotec, and rabbit anti-Ey is a gift from Patrick Callaerts. Secondary antibodies were from Molecular Probes. Imag EXPRESSION / LABELING:
|
|
Co-injection of meis1 mRNA rescues the loss of cyclin D1 expression in meis1-morphant embryos. cyclin D1 in situ hybridization of 20-hpf embryos injected with 12 ng of meis1-MO (A), or 12 ng of meis1-MO plus 360 pg of GFP-meis1 mRNA (B). GFP-meis1 mRNA is insensitive to the MO. Three representative embryos are shown for each condition. Arrowheads point to the eyes. EXPRESSION / LABELING:
|
|
Size of GFP-meis clones at two developmental stages and subcellular localization of the GFP-Meis product. (A) Size of GFP and GFP-meis1 clones at two developmental stages. The number of clones (n) for each clone type is indicated. (B) 30-hpf eye containing GFP-meis1-expressing cells counterstained with DAPI to label DNA. GFP-meis1 cells can be seen undergoing mitosis (arrowhead in the inset). (C,D) 3-dpf GFP-meis1-expressing clones can only be recovered in the photoreceptor layer. Here, the GFP-tagged Meis1 transcription factor accumulates mostly in the cytoplasm of the photoreceptors. This contrasts with its nuclear accumulation at earlier stages (see Fig. 4). The nuclear localization of Meis proteins have been shown to depend on their interaction with Pbx products (Vlachakis et al., 2001; Choe et al., 2002). Therefore, one possible cause for the cytoplasmic accumulation of GFP-Meis1 in photoreceptors might be reduced levels, or absence, of Pbx partners in these cells. In addition, Pbx proteins can be retained in the cytoplasm by direct binding to non-muscle myosin II heavy chain B (Huang et al., 2003). If this were the case in photoreceptor cells, the Meis1-Pbx complex would not be able to travel to the nucleus. Alternatively, the overexpressed GFP-Meis1 might be saturating the nuclear import machinery, causing a large fraction of this protein to remain in the cytoplasm. |

Unillustrated author statements |