- Title
-
Dual Roles of Fer Kinase Are Required for Proper Hematopoiesis and Vascular Endothelium Organization during Zebrafish Development
- Authors
- Dunn, E.M., Billquist, E.J., VanderStoep, A.L., Bax, P.G., Westrate, L.M., McLellan, L.K., Peterson, S.C., MacKeigan, J.P., Putzke, A.P.
- Source
- Full text @ Biology (Basel)
|
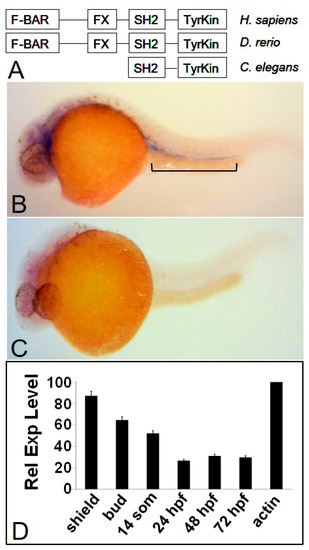
Fer kinase is conserved throughout evolution and is expressed during zebrafish embryogenesis. The zebrafish homologue of Fer kinase is highly conserved throughout eukaryotic organisms and contains the same domains as the human version (A). The zebrafish homologue of the fer kinase gene is expressed ubiquitously during early developmental stages with more specific expression emerging between 24 and 72 hpf (not shown). At 24 hpf, fer expression is observed in the intermediate cell mass (ICM) and developing brain region (B), when compared to a fer anti-sense probe (C). Quantitative PCR shows fer expression is highest during early development and tapers off by 24 hpf to three to four-fold lower levels (D). EXPRESSION / LABELING:
|
|
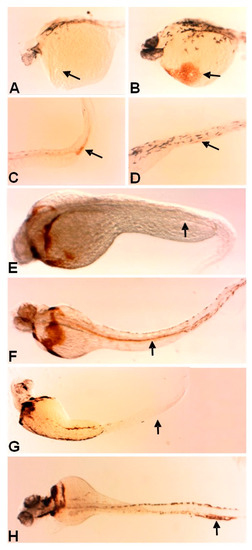
Fer knockdown results in apparent vasculature blockage and accumulation of developing hematopoietic cells. At 30 somites, fer-MO1 embryos exhibit convergence extension defects along the anterior–posterior axis with red blood cells that are pooled in the posterior ICM region (A) with none present in the pumping heart (B), when compared to wildtypes ((C,D) respectively). By 48 hpf, the lack of circulating red blood cells and empty heart chambers is emphasized in the Fer knockdown embryos (E,F) compared to wildtype embryos (G,H). Agarose gel electrophoresis of fer-reverse transcription PCR from fer-MO1 injected embryos to demonstrate the blockage of Fer splicing at exon 9 (I). |
|
The loss of Fer kinase function resulted in the loss of early anterior erythrocyte formation and the loss of circulation. o-Dianisidine staining showed the slightly higher levels of erythrocyte heme groups in the posterior trunk region of fer-MO1 embryos at 30 somites (~28 hpf) (C, control-D), but not in the anterior (A), as is observed in control embryos (B). The staining was no longer present in the posterior region at 48 and 72 hpf in fer-MO1 ((E,G) respectively) but regained anterior staining, indicating that later erythrocyte production is functional but no circulation is taking place (control F and H, 48 and 72 hpf respectively). PHENOTYPE:
|
|
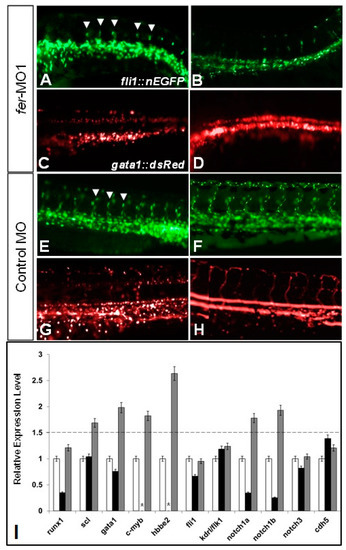
The loss of Fer kinase function resulted in intersegmental vessel (ISV) formation defects and loss of blood flow in zebrafish embryos. fer-MO1 injected embryos failed to properly organize the dorsal aorta (DA) and cardinal vein (CV) regions (A,B), and had fewer ISV structures form (white arrowheads), when compared to the control MO injected embryos (E,F). Additionally, at similar time points in the absence of Fer kinase, hematopoietic precursor cells formed in the region of the DA but failed to circulate (C,D) (control MO—(G,H)). Vasculature was visualized using fli1::nEGFP and hematopoietic cells were visualized using gata1::dsRed. In (C,D,G,H), punctate dots indicate stationary cells, while continuous lines indicate moving cells. Time points are at 30 somites (~28 hpf) for (A,C,E,G) and 48 hpf for (B,D,F,H). (Note: some cells in appear overexposed due to the abundance of overlapping cells in one region.) The quantitative PCR showed that the expression of hematopoietic precursor genes (scl, gata1, notch1a, notch1b, notch3, c-myb) were increased in the absence of Fer kinase activity (runx1 is decreased early but recovers by 30 somites), while genes expressed in the vascular endothelium remained generally unaffected (fli1, kdrl/flk1). The cell–cell adhesion complex component, cdh5 (VE-cadherin) expression was unaffected in the absence of Fer kinase (I). (black bars indicate Fer MO at 8 somites, gray bars indicate Fer MO at 28 somites, * indicate not done). The dotted line denotes the threshold for up-regulated expression, when normalized to the wildtype expression levels, which was set at a value of 1 on the y-axis. EXPRESSION / LABELING:
PHENOTYPE:
|
|
The loss of Fer kinase function resulted in vascular tubulogenesis defects. The loss of Fer kinase function decreased the diameter of the dorsal aorta (A,C) and resulted in fewer migrating cells into the ISV regions (D) at 30 somites (Control embryos (B–D). Rhodamine–Dextran injections into DA resulted in no visible dye circulation in Fer deficient embryos ((E), fli1::nEGFP, (G), Rhodamine), when compared to control embryos ((F), fli1::nEGFP, (H), Rhodamine). Confocal microscopy images demonstrated a concurrent loss of vasculature organization in the region of the DA and gain of hematopoietic precursors at 26 hpf ((I), control MO, (J), fer-MO1). The right images in (I,J) are digital cross-sections, taken approximately midway through the image; z-stacks showed no gata1::dsRed signal in the region that should be the inner-DA. The FACS analysis showed a shift in the number of gata1::dsRed cells, along with a loss of vascular endothelial population ((K), gated regions of non-overlapping, fli1a::nEGFP and gata1::dsRed cells, (L), quantitative plot showing differences in the cell population from (K) in the presence/absence of Fer kinase activity). PHENOTYPE:
|
|
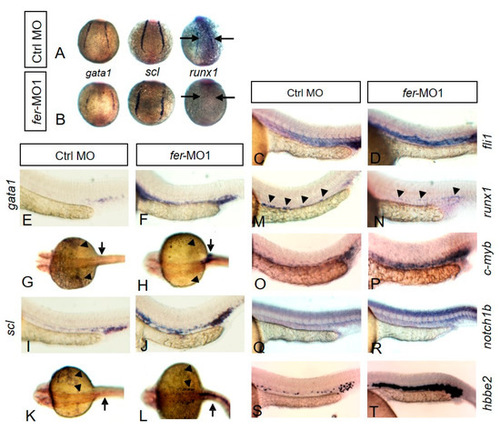
Fer kinase is required for proper expression patterns of hematopoietic genes. In situ hybridization showed the expression of gata1, scl and runx1 at the 6 somite stage in the region of the lateral plate mesoderm. Control embryos are shown in (A) while fer-MO1 embryos are shown in (B). Arrows point to the region of runx1 expression. At 30 somites, the expressions of fli1 (C,D), gata1 (E–H), scl (I–L), runx1 (M,N), c-myb (O,P), notch1b (Q,R), and hbbe2 (S,T) are shown. EXPRESSION / LABELING:
PHENOTYPE:
|
|
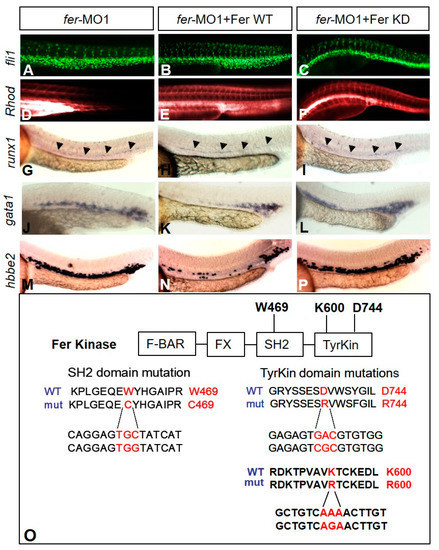
Kinase-inactive Fer rescues vascular tubulogenesis but not hematopoiesis gene expression defects. The injection of fer mRNA and fer-MO1 into fli1a::nEGFP embryos results in increased cell migration into the ISV regions ((B)—WT, (C)—kinase inactive (KD)) when compared to fer-MO1 alone (A), and rescues tube formation ((E)—WT, (F)—KD, Rhodamine-Dextran circulation after 15 min, when compared to fer-MO1 alone (D)). Wild type Fer ameliorates defects in erythrocyte expansion, as observed with runx1 (H), gata1 (K) and hbbe2 (N), while inactive Fer does not ((I,L,P) respectively). fer-MO1 alone is shown in (G,J,M) respectively. Embryos were examined at 30 somites (~28 hpf). Domains of Fer kinase in zebrafish, showing the highly conserved tryptophan and aspartic acid residues, in the SH2 and tyrosine kinase domains, respectively, are shown. Mutating either tryptophan to cysteine (W469C), aspartic acid to arginine (D744R) or lysine to arginine (K600R) resulted in the loss of kinase activity for Fer. The amino acid sequence is shown on top, with the corresponding DNA sequence changes below ((O), Fer KD expression confirmation shown in Supplementary Figure S1). |
|
Fer functions independently of Notch for erythrocyte population expansion but not intersomitic vessel formation. Co-injection of the Notch Intracellular Domain (NICD), along with wildtype (WT) or kinase-inactive (KD) Fer, showed that active Notch partially rescues vascular migration in the absence of Fer (fli1: (F)—WT, (I)—KD), but not excess erythrocyte proliferation (gata1: (D), WT, (G), KD; scl: (E), WT, (H), KD), when compared to fer-MO1 embryos containing only the NICD (J,K,L). Control MO embryos with injected NICD show normal gene expression of gata1 (A), scl (B) and fli1 (C). fer-MO1 alone—gata1 (M), scl (N) and fli1 (O). Embryos examined at 30 somites (~28 hpf). |