- Title
-
cloche, an early acting zebrafish gene, is required by both the endothelial and hematopoietic lineages
- Authors
- Stainier, D.Y.R., Weinstein, B.M., Detrich, H.W., III., Zon, L.I., and Fishman, M.C.
- Source
- Full text @ Development
|
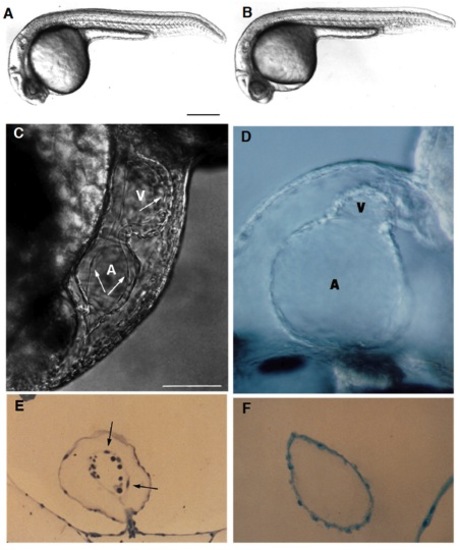
Live wild-type (A) and homozygous clom39 mutant (B) zebrafish embryos at 24 hours (all times are in hours postfertilization at 28.5°C; clo indicates the locus of the mutation, and m39 is the allele designation). At this time and at this magnification, a slight edema is noticeable in the pericardial region of the mutant embryo but no other abnormalities are apparent. Indeed, development appears normal until the onset of the heart beat by the 26-somite stage (22 hours). From that time onwards, the heart exhibits a weak beat and becomes abnormally enlarged, especially the atrium. Edema becomes very pronounced by day 2. There is no circulation, although cells are present in the trunk vessels (see Fig. 3). (C) In a normal heart at around 40 hours, the myocardium and endocardium (arrows) are clearly distinct. Endocardial cells are the thin elongated cells that form the inner layer of the heart. The clo mutant heart only has a single layer of cells (as shown in a medial view of the heart (D), see also F), determined to be the myocardium by its spontaneous contractility, and also morphologically, ultrastructurally, and biochemically, using monoclonal antibodies (mAbs) against myosin heavy chain and tropomyosin (Stainier and Fishman, 1992) (see also Figs 2 and 4). The main chambers (atrium (A) and ventricle (V)) are distinct morphologically and biochemically (using chamber specific mAbs against myosin heavy chain; data not included) (Stainier and Fishman, 1992) and contract sequentially. In the mutant heart, the chambers are dysmorphic, the atrium is abnormally enlarged and the ventricle is collapsed. Some mutant hearts undergo normal looping morphogenesis. (E) Transverse sections of the atrium of a normal heart at 3 days showing the myocardium and endocardium (arrows) as well as some blood cells (dark round cells) and of a clo mutant heart at 3 days (F) showing the presence of a single layer of cells, previously determined to be the myocardium. Scale bars, 200 mm (A,B); 50 mm (C-F). [In Fig. 1 C and E, a silent heart (sih) mutant embryo is shown as the wild-type control. Because there is no circulation in the clo mutant embryo, we wanted to distinguish the specific effects of the clo mutation from the indirect effects resulting from a lack of circulation. For this purpose, we examined the phenotype of another mutant, the sih mutant (isolated in Eugene, Oregon by C. Walker and C. Kimmel), which, as the name indicates, exhibits no heart beat, and thus no blood flow. We previously determined at the molecular level that the sih mutation is cardiac specific and is thus a good model to examine the indirect effects resulting from a lack of circulation (Stainier et al., in preparation). The sih mutant heart is morphologically similar to the wild-type heart.] PHENOTYPE:
|
|
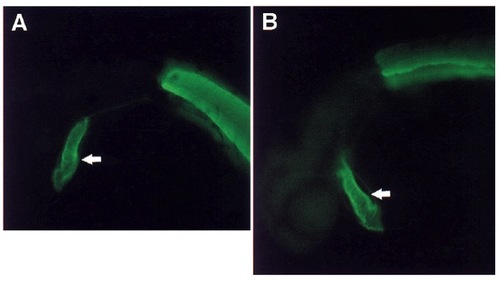
The clo mutant heart expresses normal levels of myofibrillar proteins. A number of myofibrillar proteins were examined by immunohistochemistry, including different isoforms of myosin heavy chain, troponins I and T, and tropomyosin. Comparable levels of tropomyosin as observed by immunofluorescence are seen in wild-type (A), and clo mutant (B) embryos at 36 hours. Arrows point to the heart and the other staining is skeletal muscle in the trunk. EXPRESSION / LABELING:
|
|
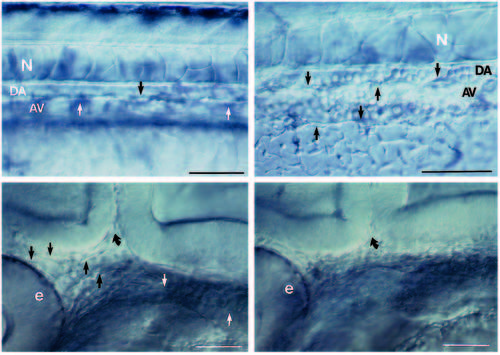
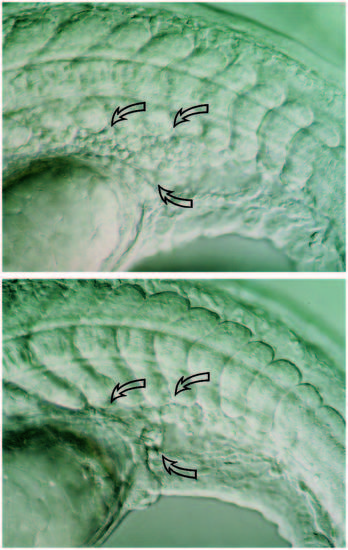
(A,B) Live embryos at 36 hours. As in wild type (A), the clo mutant (B) has blood vessels in the trunk. A shows the central vessels in the mid-trunk region; B shows the central vessels more caudally where they are easier to photograph in the mutant. Arrows point to endothelial cells. The dorsal aorta (DA) sits between the notochord (N) and the axial vein (AV). (Cells fill the trunk vessels in wild-type and mutant embryos but because they don’t circulate in the mutants, they are more clearly distinguished.) In the head, at 28 hours (which is after the initiation of the heart beat but before circulation is established in the head; Stainier et al., 1993), blood vessels are clearly seen in wild-type (C) but not in clo (D). Straight arrows point to endothelial cells; curved arrow points to the midbrain-hindbrain boundary. We also examined plastic sections throughout the head region and confirmed the Nomarski optics observations. At earlier times (and also here at 28 hours), the head mesoderm in clo appears more condensed than in wild-type, suggesting that the migrating angioblasts that normally form part of the loose head mesenchyme may in fact be missing. e, eye. Scale bars, 50 μm. PHENOTYPE:
|
|
The endocardial progenitor cells are absent from the heart region in clo mutant embryos at the time of heart tube formation. Horizontal sections of a wild-type (A,B) and a clo mutant embryo (C,D) at the 24-somite stage. In wild type, tropomyosin immunoreactive myocardial cells (arrows) surround the endocardial progenitor cells as the two primitive myocardial tubes fuse to form the definitive heart tube (Stainier et al., 1993). In clo, tropomyosin immunoreactive myocardial cells (arrows) surround a space which is conspicuously empty and somewhat collapsed. (Serial sections were examined throughout this region in several mutant embryos and no endocardial progenitor cells were seen). In C and D, a more apical region of the heart cone (i.e. a more arterial region of the heart) is shown which explains why the mutant heart appears smaller than wild type. Scale bar, 50 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
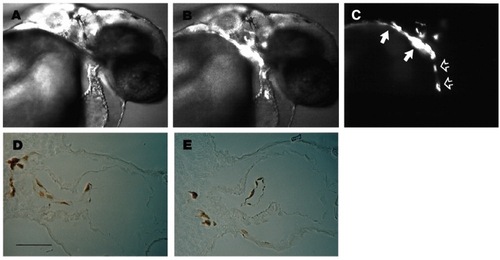
Wild-type cells can form endocardium in clo mutant embryos. Nomarski (A), Nomarski + fluorescence (B), and fluorescence (C) micrographs of the same living 36 hour mutant host embryo. Labeled cells, derived from a wild-type donor embryo, populate the outflow tract (filled arrows) and form an elongated mass of tissue in the ventricular chamber of the myocardial tube (open arrows). Transverse sections through the heart of another mutant host after horseradish peroxidase staining of the biotin dextran labeled wild-type cells (D,E) reveal that transplanted wild-type cells form a distinctly elongated cell layer, the endocardial layer, inside the heart of the host embryo. When endocardium was observed in wild-type (WT) to mutant (M) transplants, all the endocardial cells were derived from WT as assessed by immunoreactivity to biotin (n=10). Scale bar 50 μm (D,E). |
|
The number of presumptive hematopoietic stem cells is reduced in clo mutant embryos. Nomarski micrographs of living wild-type (A), and clo mutant (B) embryo around the 20-somite stage. Hematopoietic stem cells are thought to come from the posterior region of the ICM. Wild-type embryos contain a large number of round, undifferentiated cells in this region (open arrows) while clo mutants exhibit a marked reduction in the number of these cells. These presumptive stem cells are further identified as they express GATA-2 but not GATA-1 (see Fig. 7). This reduction of cells in the posterior region of the ICM is the earliest morphological phenotype observed during development and was used reliably to sort mutant embryos at an early stage. PHENOTYPE:
|
|
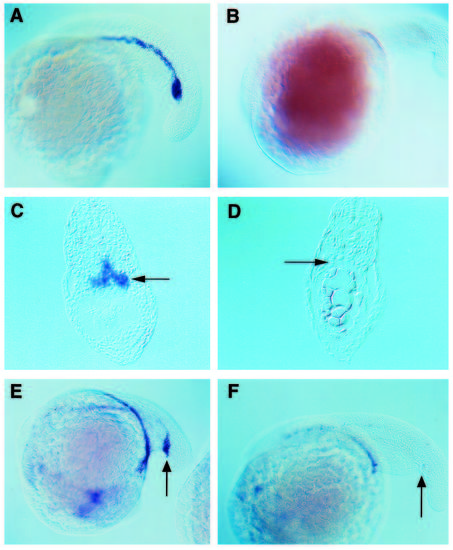
clo mutant embryos do not express GATA-1 or GATA-2 in their hematopoietic tissues. In situ hybridization with a GATA-1 or GATA-2 antisense RNA probe was performed on wild-type embryos (A,C,E) and on clo mutants (B,D,F). (A-D) GATA-1 staining at the 22-somite stage. In wild-type embryos, GATA-1 expression is originally found in two longitudinal chords of cells that extend from the mid-trunk region to just beyond the yolk tube and pronephric ducts. These cell populations will fuse at the midline to form the ICM as seen in whole mounts (A) and transverse sections of the tail region (C). clo mutants fail to express GATA-1 (B,D). (E,F) GATA-2 staining of 20-somite stage embryos. GATA-2 is normally expressed from an early stage in all hematopoietic progenitors including the presumptive stem cells of the posterior ICM (E, arrow). In clo mutants, the hematopoietic tissues do not express GATA-2 (F). GATA-2 is also expressed in certain neural tissues (Detrich et al., 1995) and this expression is normal in mutant embryos (data not included). EXPRESSION / LABELING:
|