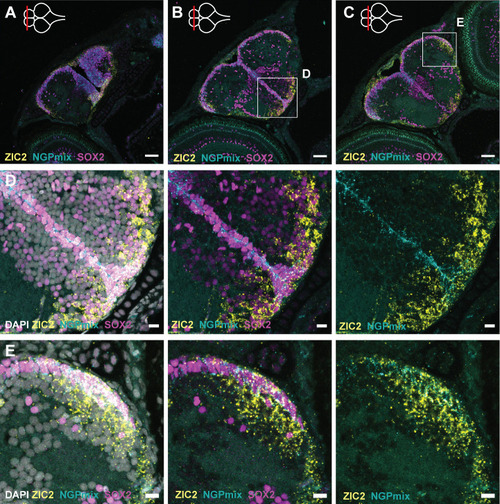
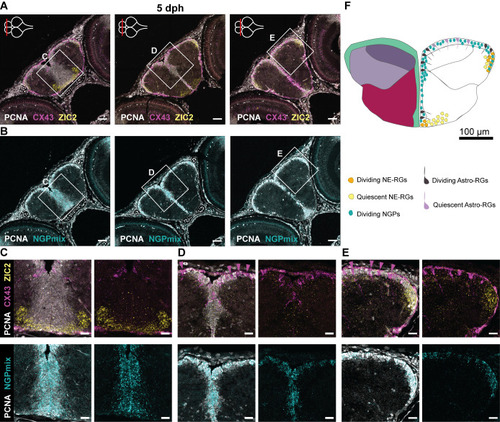
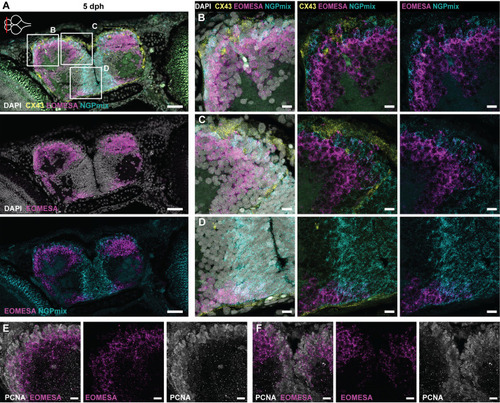
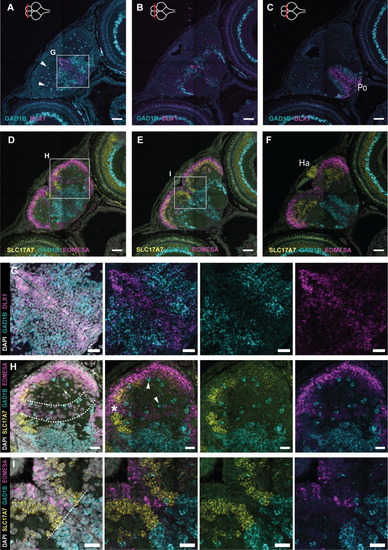
- Title
-
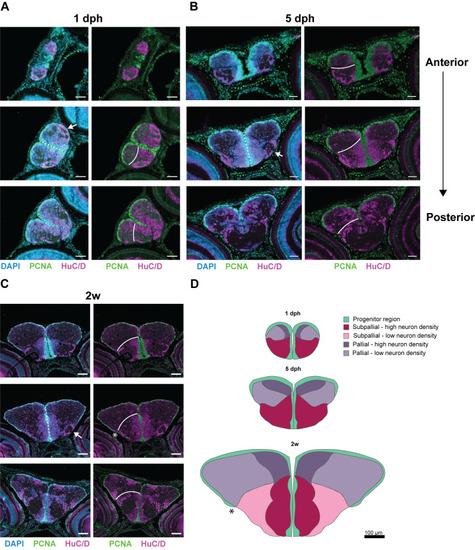
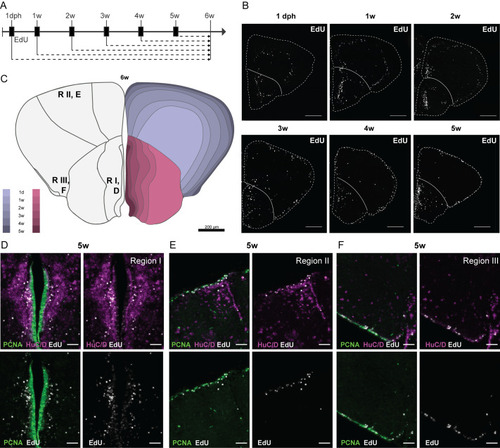
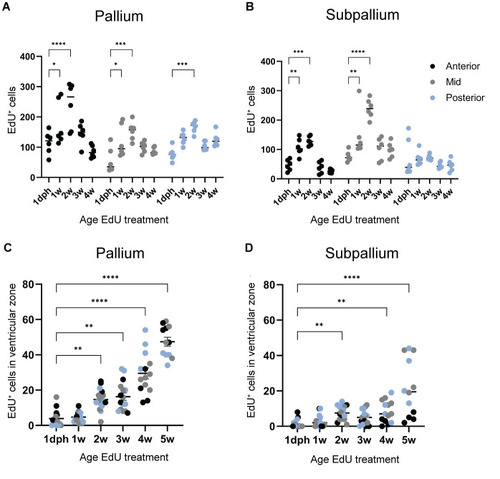
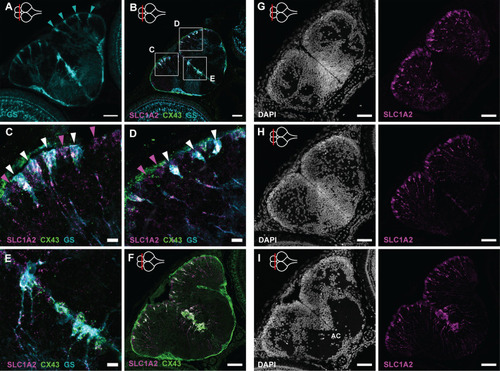
Early-life growth and cellular heterogeneity in the short-lived African turquoise killifish telencephalon
- Authors
- Zandecki, C., MariŽn, V., Ayana, R., Van Houcke, J., Arckens, L., Seuntjens, E.
- Source
- Full text @ Biol. Open
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|