- Title
-
Peds1 deficiency in zebrafish results in myeloid cell apoptosis and exacerbated inflammation
- Authors
- Arroyo, A.B., Tyrkalska, S.D., Bastida-Martínez, E., Monera-Girona, A.J., Cantón-Sandoval, J., Bernal-Carrión, M., García-Moreno, D., Elías-Arnanz, M., Mulero, V.
- Source
- Full text @ Cell Death Discov
|
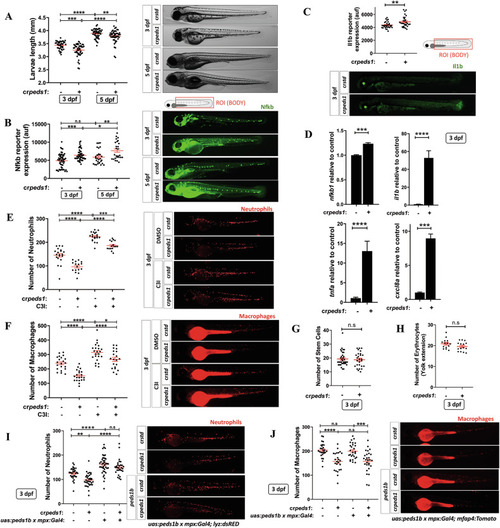
Peds1 deficiency promotes a proinflammatory stage with increased neutrophil and macrophage apoptosis. One-cell stage zebrafish eggs were microinjected with |
|
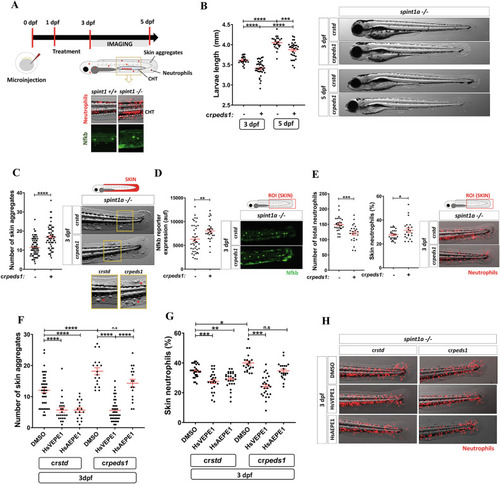
Peds1 deficiency exacerbates chronic skin inflammation. |
|
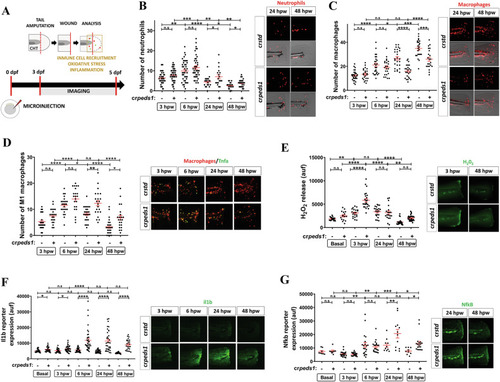
Peds1 deficiency impairs inflammation resolution and causes aberrant immune cells recruitment in a sterile tail injury model. |
|
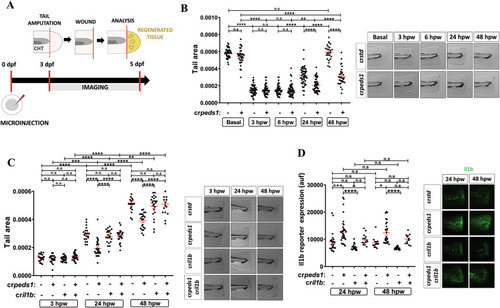
Peds1 deficiency hampers tissue regeneration. |
|
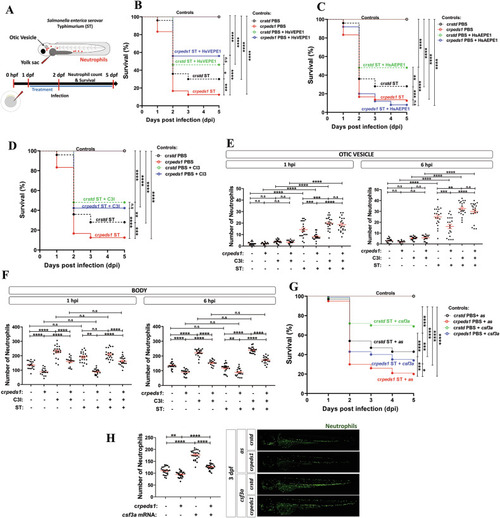
Peds1 deficiency increased susceptibility to bacterial infection. |
|
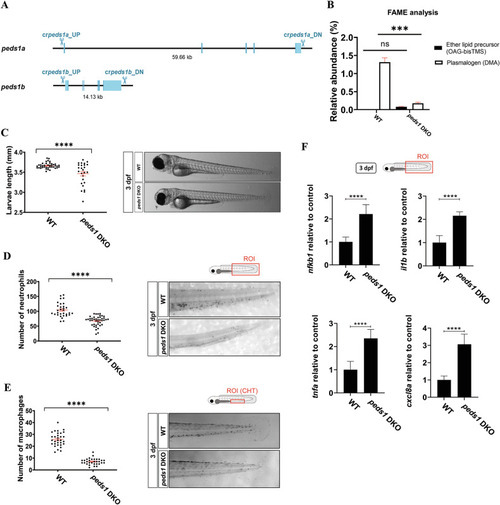
Peds1a/b DKO line shows developmental delay, neutropenia, monocytopenia and exacerbated inflammation. |
|
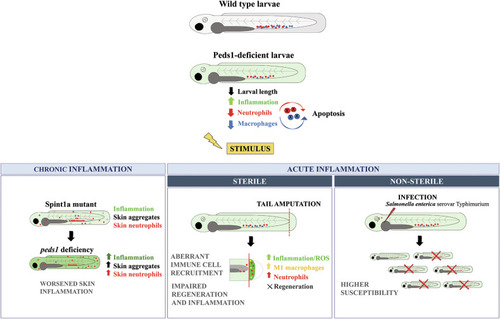
Model showing the effect of inhibiting plasmalogen synthesis under basal and different inflammatory conditions. Inhibition of plasmalogen synthesis promotes a proinflammatory stage with increased neutrophil and macrophage apoptosis. Plasmalogen deficiency also exacerbates chronic skin inflammation, impairs inflammation resolution and tissue regeneration, and results in increased susceptibility to infection. |