- Title
-
Specific Activation of Yamanaka Factors via HSF1 Signaling in the Early Stage of Zebrafish Optic Nerve Regeneration
- Authors
- Sugitani, K., Mokuya, T., Homma, S., Maeda, M., Konno, A., Ogai, K.
- Source
- Full text @ Int. J. Mol. Sci.
|
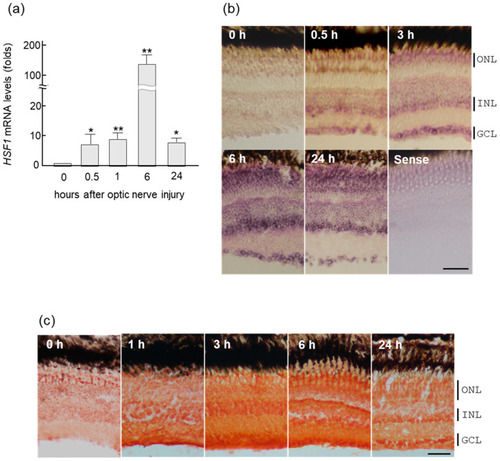
Upregulation of HSF1 (heat shock factor 1) mRNA in zebrafish retina after ONI (optic nerve injury). (a) HSF1 mRNA expression levels after ONI were determined using quantitative real-time PCR. (b) In situ hybridization of HSF1 in the zebrafish retina after nerve injury. HSF1 mRNA started to increase in the retina for 0.5 h and peaked at 6 h after ONI. Its localization was first seen in the GCLs (ganglion cell layers) and after the INLs (inner nuclear layers). Then, these signals spread to all nuclear layers including the ONLs (outer nuclear layers) at 6 h and slightly decreased at 24 h after ONL. (c) Immunohistochemical staining of HSF1 in the zebrafish retina after ONI. Significant immunostaining peaked at 3 to 6 h in all nuclear layers after ONI. Data are expressed as the mean ± SEM of five independent experiments and analyzed by one-way ANOVA, followed by Scheffe’s multiple comparisons. Statistical significance was set at * p < 0.05 or ** p < 0.01. Scale bar = 50 μm. |
|
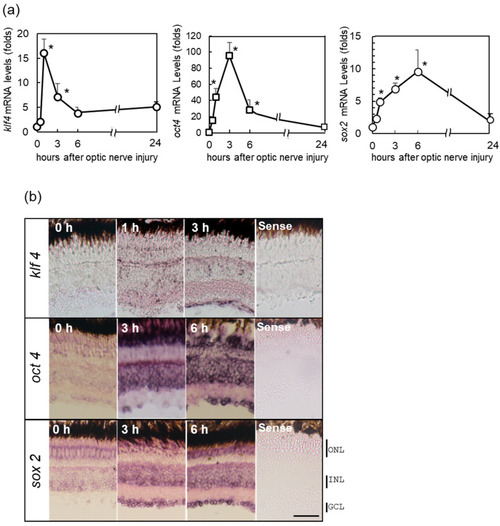
Upregulation of the Yamanaka factors (OSK) in zebrafish retina after ONI. (a) mRNA expression levels of OSK after ONI were determined by quantitative real-time PCR (left, klf4; center, oct4; right, sox2). (b) In situ hybridization of OSK in zebrafish retina after ONI. Klf4 mRNA expression started to increase at 1 h and localized to the GCLs at 3 h after ONI. Oct4 mRNA signal was observed in the GCL and strongly in the INL and ONL at 3 h, but this strong signal was seen in all nuclear layers at 6 h after ONI. Sox2 mRNA expression was observed in all nuclear layers 3 h after ONI and more prominent at 6 h. No positive signals could be seen with the sense probe (Sense). Five to six experiments were repeated with different retinas under each experimental condition and produced the same results. Data are expressed as the mean ± SEM and analyzed by one-way ANOVA, followed by Scheffe’s multiple comparisons. Statistical significance was set at * p < 0.05. Scale bar = 50 μm. |
|
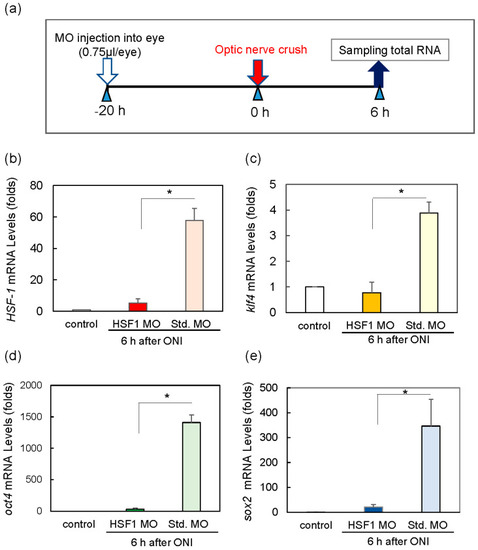
Treatment of HSF1 MO (morpholino) significantly reduced the mRNA expression of Klf4, Oct4, and Sox2 6 h after ONI. (a) HSF1 MO or standard MO (Std. MO) was injected intraocularly 20 h before ONI. (b) HSF1 MO-treated group suppressed HSF1 mRNA expression compared to the Std. MO-treated group. Under these conditions, the mRNA expression of Klf4 (c), Oct4 (d), and Sox2 (e) was inhibited compared to the control (Std. MO) groups. Five experiments were repeated under each experimental condition. Data are expressed as the mean ± SEM of independent experiments and analyzed by one-way ANOVA, followed by Scheffe’s multiple comparisons. Statistical significance was set at * p < 0.05. |
|
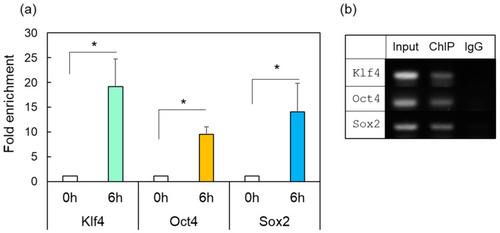
ChIP-enriched DNA was prepared using preimmune serum (IgG) or anti-HSF1 antibody from the control (0 h) or damaged zebrafish retina after ONI (6 h). (a) The immunoprecipitated DNA of Klf4, Oct4, and Sox2 were analyzed by real-time PCR. Each ChIP signal was divided by the no-antibody signals (IgG), representing the ChIP signals as the fold increase in signals relative to the background signals. (b) Gel electrophoresis image using the ChIP samples. The input was used as an internal positive control for the ChIP assay. Five to six experiments were repeated with different retinas under each experimental condition. Data are expressed as the mean ± SEM of independent experiments and analyzed by one-way ANOVA, followed by Scheffe’s multiple comparisons. Statistical significance was set at * p < 0.05. |