- Title
-
Embryonic Hyperglycemia Delays the Development of Retinal Synapses in a Zebrafish Model
- Authors
- Shrestha, A.P., Saravanakumar, A., Konadu, B., Madireddy, S., Gibert, Y., Vaithianathan, T.
- Source
- Full text @ Int. J. Mol. Sci.
|
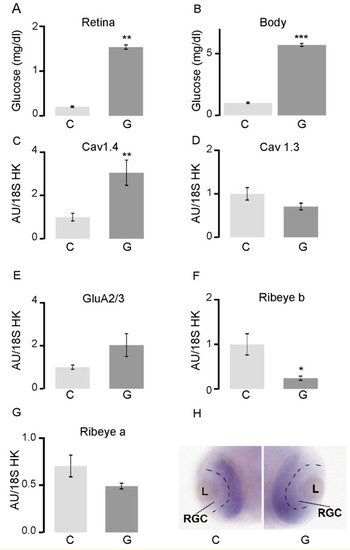
In zebrafish larvae, hyperglycemia results in altered transcription of retinal synaptic genes and localization of synaptic proteins. (A) Glucose concentration at 48 hpf in the retina and (B) in the rest of the body in embryos exposed to 4% glucose 24–48 hpf; (C–G) RT-qPCR analysis of the whole retina in embryos exposed to 4% glucose 24–48 hpf. All transcript levels were normalized to those of 18S rRNA. The levels of cav1.4a transcripts increased, those of cav1.3a, GluR2/3, and ribeye a remained unchanged, while those of ribeye b decreased in retinas exposed to glucose, relative to controls; (H) In situ hybridization for ribeye a in the transverse sections in the center of the eye 48 hpf in control embryos (C) and those exposed to 4% glucose (G). The separation between the already defined retinal ganglia cell layer and the developing remainder of the retinal layer is marked by a black dashed line. * p < 0.05; ** p < 0.01; *** p < 0.001. Abbreviations: C, control; G, exposed to glucose; GluR2/3, glutamate receptor; hpf, hours post-fertilization; rRNA, ribosomal RNA; RT-qPCR, reverse transcription quantitative PCR; L, lens; RGC, retinal ganglion cells. |
|
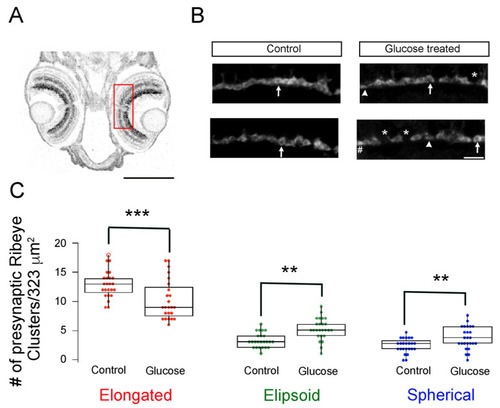
The maturation of synaptic ribbons is delayed in hyperglycemic zebrafish larvae. (A) Imaging region of the retina is illustrated with a ROI (red) placed on the transverse section of 5 dpf larvae. Scale bar, 200 μm; (B) Maximal intensity projection of confocal z-stacks of retinal sections from control (left panels) and hyperglycemic larvae (right panels) immunostained with fluorescently labeled antibodies specific for ribeye/CtBP2. Mature (elongated, arrow) and immature (ellipsoid, arrowhead or spherical, #) ribbon morphologies are indicated. * Denotes the gaps between ribbons. Scale bar, 5 μm; (C) Quantitative analyses of ribbon morphologies in retinal sections stained for ribeye/CtBP2 were categorized according to morphology, as described in Materials and Methods: mature (elongated, red) and immature (ellipsoid, green or spherical, blue) morphologies were noted and are shown as box-and-whisker plots. Boxes show all values, while whiskers indicate minimum and maximum values. Data are presented as mean values ± SEM. The total number of profiles examined was 521 control larvae and 388 glucose-treated larvae in groups of 50 larvae; the retinas from each of the larvae were processed independently. ** p < 0.01; *** p < 0.001. Abbreviations: CtBP2, C-terminal binding protein 2; dpf, days post-fertilization; ROI, region of interest; SEM, standard error of the mean. |
|
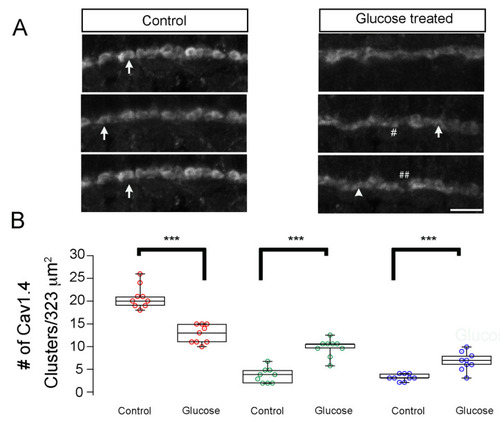
Hyperglycemia leads to developmental changes in Cav1.4/cacna1f accumulation in the OPL of zebrafish larvae. (A) Transverse retinal sections from 5 dpf control (left) and hyperglycemic (right) larvae immunostained with fluorescently labeled antibodies specific for Cav1.4/cacna1f. Maximal intensity projections are shown. Mature (elongated, arrow) and immature (ellipsoid, closed arrows or spherical, #) ribbon morphologies are indicated. ## Denotes the gaps between Cav1.4 proteins. Scale bar, 5 µm. (B) Quantitative analyses of IHC for Cav1.4/cacna1f were performed similarly for ribeye/CtBP2 and were categorized according to morphology, as described in Materials and Methods: mature (elongated, red) and immature (ellipsoid, green or spherical, blue) morphologies were noted and are shown as box-and-whisker plots. Boxes show all values, while whiskers indicate minimum and maximum values. Data are presented as mean values ± SEM. *** p < 0.001. Abbreviations: Cav1.4/cacna1f, voltage-dependent calcium channel; dpf, days post-fertilization; IHC, immunohistochemistry; SEM, standard error of the mean. |
|
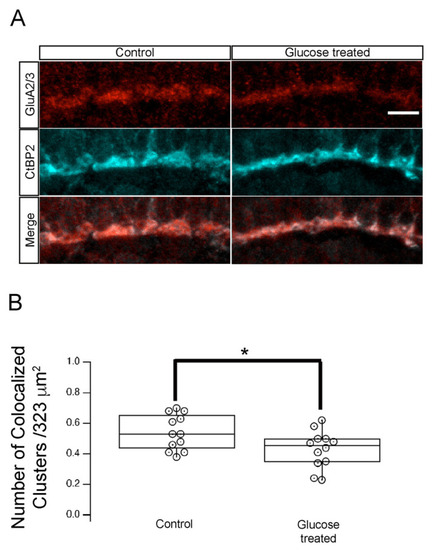
Hyperglycemia altered the synapse between photoreceptor and GluR2/3-containing neurons in the retinal INL. (A) Transverse retinal sections from 5 dpf control (left panels) and hyperglycemic (right panels) larvae were immunostained with fluorescently labeled antibodies specific for CtBP2 (red, top) or GluR2/3 (cyan, middle); overlays of CtBP2 and GluR2/3 signals are shown (merge; bottom). Maximal intensity projections are shown. Scale bar, 5 µm. We observed co-localization of ribeye/CtBP2 and GluR2/3 proteins in the OPL. (B) Quantitative analyses of IHC for CtBP2 and GluR2/3 in the OPL shown by box-and-whisker plots that indicate the number of colocalized clusters from control and hyperglycemic larvae. Scale bar, 5µm. Boxes indicate interquartile ranges, while whiskers indicate range of maximal and minimal values. Data are presented as mean values ± SEM. * p < 0.05. Abbreviations used: CtBP2, C-terminal binding protein 2; dpf, days post-fertilization; GluR2/3, glutamate receptor 2/3; INL, inner nuclear layer; OPL, outer plexiform layer. |
|
Hyperglycemic zebrafish larvae retained photoreceptor ribbon synapses with postsynaptic densities (PSDs) in INL dendrites. (A)Transverse retinal cross-sections from 5 dpf control (left panels) or hyperglycemic (right panels) larvae were double immunostained with fluorescently labeled antibodies specific for the postsynaptic marker MAGUK (magenta, top) or Cav1.4/cacna1f (cyan, middle); overlays of MAGUK and Cav1.4 labeling are shown (merge, bottom). Maximal intensity projections are shown; arrowheads indicate potential areas of colocalization. Scale bar, 5 µm. (B) Quantitative analyses of IHC for MAGUK and Cav1,4 colocalization are indicted by box-and-whisker plots. Boxes indicate interquartile ranges, while whiskers indicate range of maximal and minimal values. Scale bar, 5 µm. Abbreviations used: Cav1.4/cacna1f, voltage-dependent calcium channel; Dpf, days post-fertilization; INL, inner nuclear layer; MAGUK, membrane-associated guanylate kinase; PSD, postsynaptic densities; SEM, standard error of the mean. |
|
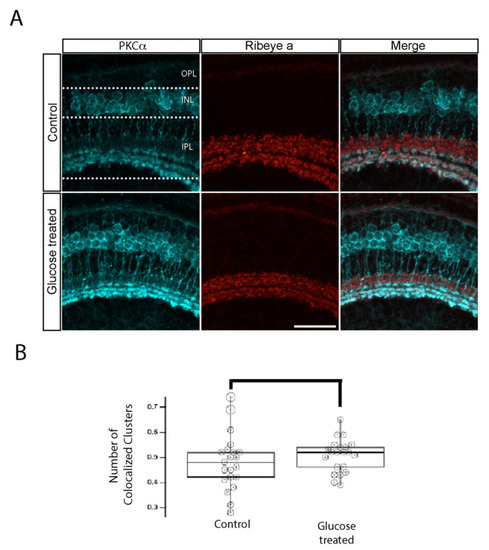
IPL ribbon synapses of bipolar cells are preserved in hyperglycemic larvae. (A) Transverse retinal sections from 5 dpf control (top) and hyperglycemic (bottom) larvae were double immunostained with fluorescently labeled antibodies specific for the rod bipolar cell marker PKCα (cyan; left panels) or ribeye a (red; middle panels); also shown is the overlay of PKCα and ribeye a labeling (merge; right panels). Maximal intensity projections are shown, and the relative positions of the OPL, INL, and IPL are indicated. Scale bar, 5 µm. The expression pattern of PKCα-labeled rod bipolar cell neurons, synaptic ribbons in the IPL layer, and the number of ribbon synapses in rod bipolar cells are normal in 5 dpf hyperglycemic larvae. (B) Quantitative analyses of IHC for colocalization of PKCα and ribeye a are indicated by box-and-whisker plots. Boxes indicate median values, and whiskers indicate 5th–95th percentile values of pooled data from 56 larvae. The data represent the total ribeye a labeling in PKCα-labeled terminals (p > 0.05). Abbreviations used: Dpf, days post-fertilization; PKCα, protein kinase C alpha; SEM, standard error of the mean. |
|
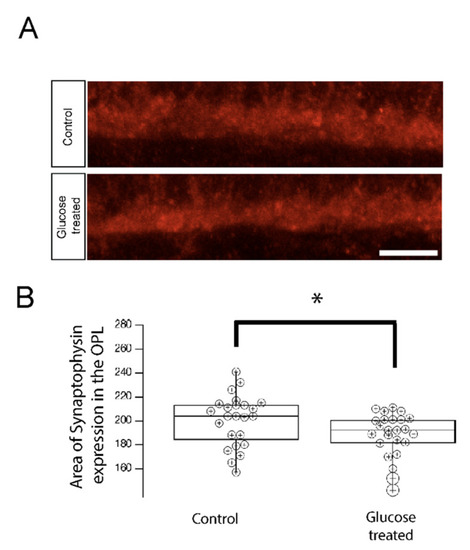
Synaptophysin accumulation in the OPL is reduced in 5 dpf hyperglycemic larvae. (A) Transverse retinal sections from 5 dpf control and hypoglycemic larvae were immunostained with fluorescently labeled antibodies specific for the synaptic vesicle marker synaptophysin. Maximal intensity projections are shown. Scale bar, 5 µm. Synaptophysin accumulation was reduced in hyperglycemic larvae relative to that in control larvae. (B) Quantitative analyses of IHC for synaptophysin accumulation in control and hyperglycemic larvae are indicated by box-and-whisker plots. Boxes indicate median value ranges, while whiskers indicate 5th–95th percentile values. Data represent the average value of the area of expression in 6–9 animals obtained from 3–5 independent experiments we sampled per condition, shown as individual data points. Scale bar, 5 µm. * p < 0.05; two-tailed t-test. Abbreviations used: dpf, days post-fertilization; SEM, standard error of the mean. |