- Title
-
A conserved residue in the P2X4 receptor has a nonconserved function in ATP recognition
- Authors
- Chen, P.F., Ma, X.F., Sun, L.F., Tian, Y., Fan, Y.Z., Li, P., Xiao, Z., Zhu, M.X., Guo, C.R., Li, C., Yu, Y., Wang, J.
- Source
- Full text @ J. Biol. Chem.
|
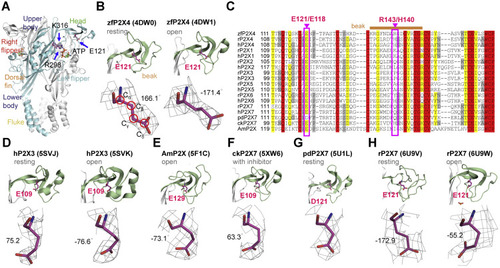
Figure 1. Side-chain orientations of E121 in zfP2X4 and equivalent residues in other P2X receptors. A, residues E121, R298, and K313 highlighted in the open structure of zfP2X4 (Protein data Bank [PBD] ID: 4DW1). The different domains are named head, dorsal fin (DF), left flipper (LF), right flipper (RF), body and fluke, respectively. B, zoom-in view of the location of E121 (upper) and 2Fo–Fc omit map for E121 (lower) in zfP2X4 at the resting (PDB ID: 4DW0, left) and open (right) states. Red circles indicate the atoms chosen to define the dihedral angle χCα–Cβ–Cγ–Cδ. C, the sequence alignment of the head domain in P2X family. Pink boxed regions highlight the highly conserved residue E121/E118 and nonconserved residue R143/H140 (zfP2X4/rP2X4 numbering) in P2X receptors. Sand bar indicates residues in the beak region. D–G, side-chain orientations of E109 in the apo (PDB ID: 5SVJ) and open (PDB ID: 5SVK) crystal structures of hP2X3, E129 in the open structure of AmP2X (PDB ID: 5F1C) (E), E109 in the crystal structure of ckP2X7 in complex with inhibitor (PDB ID: 5XW6) (F) and D121 in the apo crystal structure of pdP2X7 (PDB ID: 5U1L) (G). H, the side-chain orientation of E121 in the cryo-EM structure of rP2X7 at the resting (PDB ID: 6U9V) and open (PDB ID: 6U9W) states. rP2X4, rat (Rattus norvegicus) P2X4; zfP2X4, zebrafish (Danio rerio) P2X4. |
|
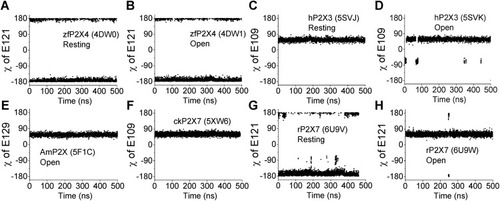
Figure 2. Molecular dynamics (MD) simulations used to test the stability of dihedral angle χCα–Cβ–Cγ–Cδ in various P2X structures. A–H, the fluctuation of dihedral angle χCα–Cβ–Cγ–Cδ during 0.5-μs MD simulations of zfP2X4 (A, resting; B, open), hP2X3 (C, resting; D, open), AmP2X (E, open), ckP2X7 (F, with bound inhibitor), and rP2X7 (G, resting; H, open). The trajectories were sampled every 200 ps. AmP2X, gulf coast tick (Amblyomma maculatum) P2X; ckP2X7, chicken (Gallus gallus domesticus) P2X7; hP2X3, human (Homo sapiens) P2X3; rP2X7, rat (Rattus norvegicus) P2X7; zfP2X4, zebrafish (Danio rerio) P2X4. |
|
Figure 3. Altered apparent ATP affinities of ATP in P2X mutants. A–I, concentration–response curves of ATP in WT and equivalent alanine substitutions in zfP2X4 (A), rP2X4 (B), hP2X3 (C), AmP2X (D), ckP2X7 (E), pdP2X7 (F), hP2X7 (G), hP2X1 (H), and hP2X2 (I). The solid lines are fitted by Hill equation (mean ± SD, n = 3–7, EC50 = 1426 ± 733 and 89.6 ± 18.1 μM, nH = 0.94 ± 0.16 and 1.02 ± 0.18 for zfP2X4WT and zfP2X4E121A; EC50 = 7.65 ± 1.56 and 45.92 ± 9.05 μM, nH = 1.20 ± 0.22 and 1.11 ± 0.15 for rP2X4WT and rP2X4E118A; EC50 = 0.45 ± 0.07 and 0.58 ± 0.07 μM, nH = 0.94 ± 0.16 and 1.02 ± 0.18 for hP2X3WT and hP2X3E109A; EC50 = 43.5 ± 4.7 and 32.9 ± 4.2 μM, nH = 1.14 ± 0.11 and 0.99 ± 0.09 for AmP2XWT and AmP2XE129A; EC50 = 4.77 ± 0.69 and 5.31 ± 1.26 μM, nH = 1.14 ± 0.16 and 0.91 ± 0.16 for ckP2X7WT and ckP2X7E109A; EC50 = 95.7 ± 17.6 and 96.6 ± 10.9 μM, nH = 1.44 ± 0.32 and 1.53 ± 0.24 for pdP2X7WT and pdP2X7D121A; EC50 = 496.7 ± 44.2 and 360.5 ± 54.3 μM, nH = 1.71 ± 0.19 and 1.34 ± 0.20 for hP2X7WT and hP2X7E121A; EC50 = 0.86 ± 0.16 and 0.92 ± 0.17 μM, nH = 1.04 ± 0.16 and 1.46 ± 0.33 for hP2X1WT and hP2X1E119A; EC50 = 13.16 ± 1.97 and 12.92 ± 1.68 μM, nH = 1.15 ± 0.17 and 1.26 ± 0.15 for hP2X2WT and hP2X2E127A, respectively). Imax was defined as the saturating ATP-induced current for WT and equivalent alanine-substituted P2X receptors, except for the maximum concentration of ATP (3 mM) used for zfP2X4E121A. J and K, representative current traces (J) and pooled data (K) for WT and equivalent alanine substitution of P2X receptors. The scatter of each open circle represents each measurement, n = 7, 4, 5, 5, 3, 4, 4, 5, 6, 5, 5, 5, 5, 5, 5, 6, 8, and 8, from left to right, respectively. AmP2X, gulf coast tick (Amblyomma maculatum) P2X; ckP2X7, chicken (Gallus gallus domesticus) P2X7; hP2X3, human (Homo sapiens) P2X3; pdP2X7, giant panda (Ailuropoda melanoleuca) P2X7; rP2X4, rat (Rattus norvegicus) P2X4; zfP2X4, zebrafish (Danio rerio) P2X4. |
|
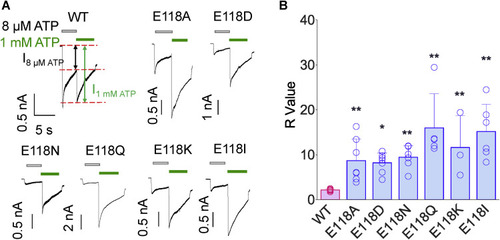
Figure 4. Role of E118 in determining the apparent affinity of rP2X4. A and B, representative current traces (A) and pooled data (B) for WT and rP2X4 mutants. About 8 μM ATP was first applied (gray bars) and then switched to 1 mM ATP (green bars). R values are derived from the ratio of 1 mM ATP-induced current to 8 μM ATP-induced current. Open circles of each scatter represent each measurement through whole-cell recordings, n = 15, 7, 7, 6, 5, 3, and 6, from left to right, respectively. ∗p < 0.05, ∗∗p < 0.01 versus WT, one-way ANOVA with Bonferroni post-test (F(6, 42) = 11.54, p < 0.0001). rP2X4, rat (Rattus norvegicus) P2X4. |
|
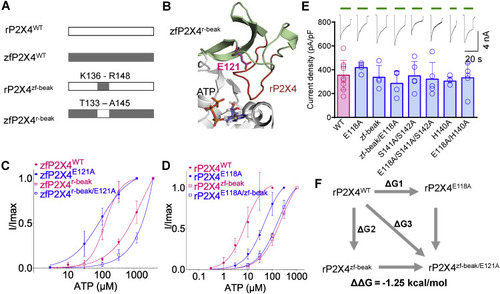
Figure 5. The role of E118 in the apparent affinity of ATP correlates with the sequence of the beak region in rP2X4. A, chimera construction of rP2X4 and zfP2X4. B, zoom-in view of the head and beak regions of the chimera zfP2X4r-beak (homology model). The beak region of zfP2X4 (pale green) is replaced by equivalent residues of rP2X4 (red). C and D, concentration–response curves of ATP in zfP2X4 WT and mutant receptors (C) and rP2X4 WT and mutant receptors (D). The solid lines are fitted by Hill equation (mean ± SEM, n = 3–6, EC50 = 232.3 ± 50.3 and 161.7 ± 26.5 μM, nH = 1.02 ± 0.11 and 1.07 ± 0.11 for rP2X4zf-beak and rP2X4zf-beak/E118A, respectively). The data of zfP2X4WT, zfP2X4E121A, rP2X4WT, and rP2X4E118A are the same as those shown in Figure 3, A and B and are shown here as controls. E, representative current traces and pooled data for rP2X4 WT and mutant receptors. The scatter of each open circle represents each measurement, n = 9, 4, 4, 4, 5, 5, 5, and 5, from left to right, respectively. One-way ANOVA with Bonferroni post-test (F(7, 33) = 0.5623; p = 0.7807). F, double-mutant cycle between E118 and the beak region of rP2X4. rP2X4, rat (Rattus norvegicus) P2X4; zfP2X4, zebrafish (Danio rerio) P2X4. |
|
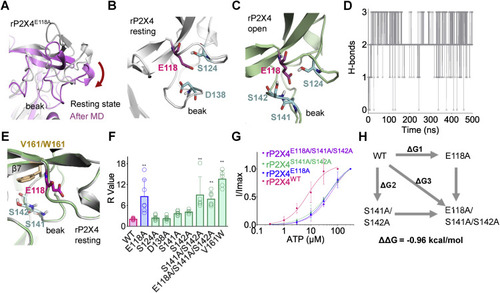
Figure 6. E118 interacts with S141/S142 in the beak region of rP2X4 receptors. A, downward motion of the head domain during 0.5-μs molecular dynamics (MD) simulation of rP2X4E118A in the resting state. B, polar amino acids of the head domain near E118 (within 5 Å) in rP2X4 in the resting (B) and open (C) states. D, the H-bond interactions between E118 and S141/S142 during 0.5-μs MD simulation of open structure of rP2X4. The trajectory was sampled every 200 ps. E, superimposed homology models of V161 and its tryptophan substitution (W161) to show the position of this residue (at rigid β7) near to S141, S142, and E118. F, summary of R values of WT and rP2X4 mutants. n = 15, 7, 8, 7, 7, 5, 5, 6, and 5, from left to right, respectively. One-way ANOVA with Bonferroni post-test (F(8, 56) = 19.98, p < 0.0001). The data on WT and E118A are the same as those displayed for Figure 5E. G, concentration–response curves of ATP in rP2X4WT, rP2X4E118A, rP2X4S141A/S142A, and rP2X4E118A/S141A/S142A. The solid lines are fitted by Hill equation (mean ± SEM, n = 3–5, EC50 = 51.5 ± 16.1 and 59.6 ± 13.2 μM, nH = 0.92 ± 0.14 and 1.07 ± 0.14 for S141A/S142A and S141A/S142A/E118A, respectively). The data of rP2X4WT and rP2X4E118A are the same as those displayed for Figure 4B and are shown here as controls. H, double-mutant cycle analysis between E118 and S141/S142. rP2X4, rat (Rattus norvegicus) P2X4. |
|
Figure 7. H140 mediates the coupling of E118 to the ATP recognition of rP2X4. A, superimposed apo and open structures of rP2X4 to show the downward motions of the head domain and beak region, as well as the H140s moving closing to the bound ATP. Arrows highlight bound ATP–induced allostery. The yellow-dashed line indicates the distance between the Cα of H140 and N9 atom of ATP. B and C, superimposition of the initial open structure and averaged conformations of rP2X4WT (B) and rP2X4E118A (C) after 0.5-μs molecular dynamics (MD) simulations. Red arrows indicate the conformational changes during MD simulations. D and E, fluctuations of the dihedral angle of H140 (D) and distance between ATP and H140 (E) during 0.5-μs MD simulations of rP2X4WT and rP2X4E118A. The trajectories were sampled every 200 ps. F, the ATP concentration–response curves of rP2X4WT, rP2X4E118A, rP2X4H140A, and rP2X4E118A/H140A (mean ± SEM, n = 3–5, EC50 = 53.3 ± 9.1 and 55.1 ± 14.4 μM, nH = 1.24 ± 0.16 and 0.99 ± 0.15 for H140A and E118A/H140A, respectively). The data on rP2X4WT and rP2X4E118A are the same as those displayed for Figure 4B. G, double-mutant cycle analysis between E118 and H140A. rP2X4, rat (Rattus norvegicus) P2X4. |
|
Figure 8. Molecular dynamics (MD) simulations of hP2X3WT, AmP2XWT, and their equivalent mutants. A, distance measurements between ATP and P128 during 0.5-μs MD simulations of hP2X3WT and hP2X3E109A. B and C, superimpositions of initial open structure and conformations after MD simulations of hP2X3WT and hP2X3E109A. The dashed lines indicate the distance between the Cα of P128 and N9 atoms of ATP. D, distance measurements between ATP and I115 during 0.5-μs MD simulations of AmP2XWT and AmP2XE129A. E and F, superimpositions of initial open structure and snapshots after MD simulations of AmP2XWT and AmP2XE129A. The trajectories were sampled every 200 ps. AmP2X, gulf coast tick (Amblyomma maculatum) P2X; hP2X3, human (Homo sapiens) P2X3. |