- Title
-
Selective Requirements for Vascular Endothelial Cells and Circulating Factors in the Regulation of Retinal Neurogenesis
- Authors
- Dhakal, S., Rotem-Bamberger, S., Sejd, J.R., Sebbagh, M., Ronin, N., Frey, R.A., Beitsch, M., Batty, M., Taler, K., Blackerby, J.F., Inbal, A., Stenkamp, D.L.
- Source
- Full text @ Front Cell Dev Biol
|
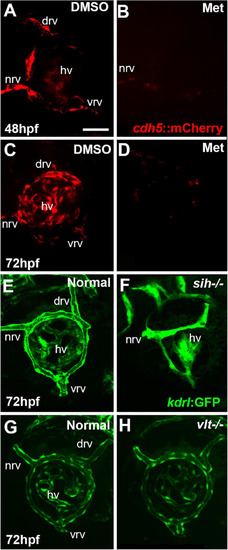
Ocular vasculature of cardiovascular disruption model systems. |
|
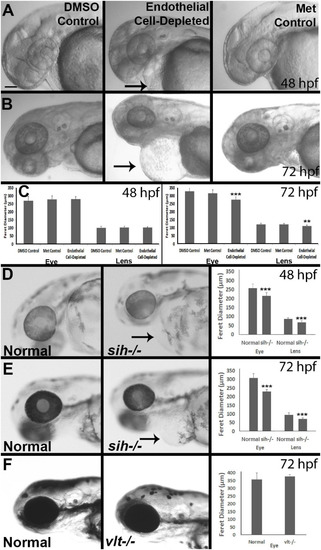
Eye and lens sizes in cardiovascular disruption model systems. |
|
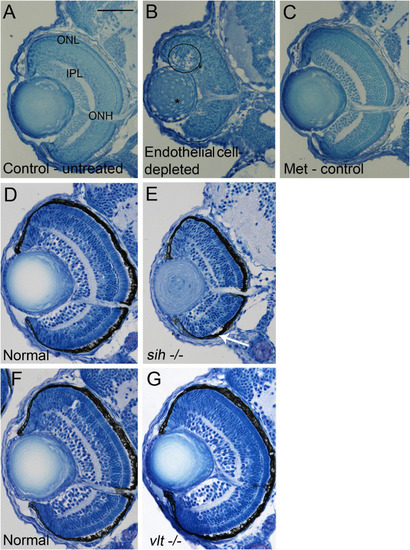
Retinal histology in cardiovascular disruption model systems. |
|
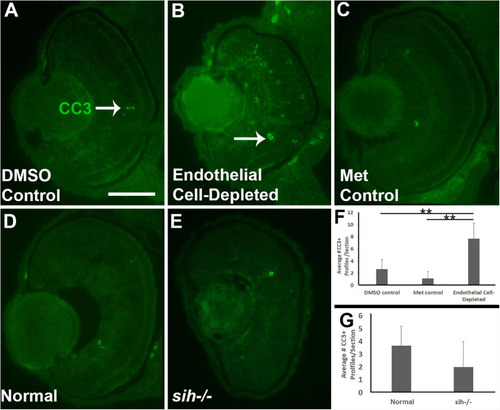
Retinal cell death in cardiovascular disruption model systems. |
|
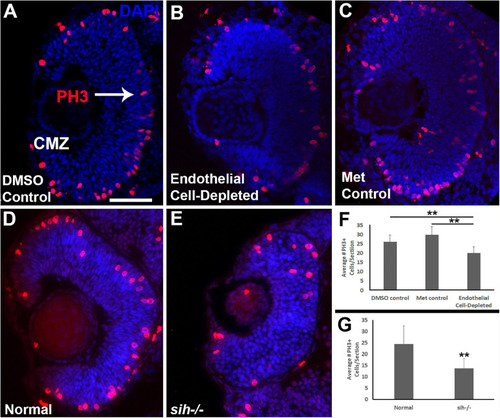
Retinal cell proliferation in cardiovascular disruption model systems. |
|
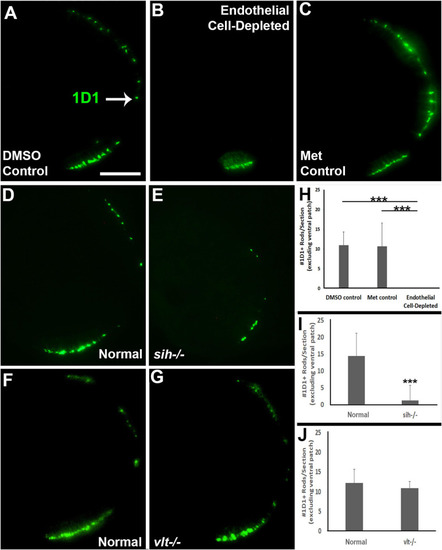
Rod photoreceptors in cardiovascular disruption model systems. |
|
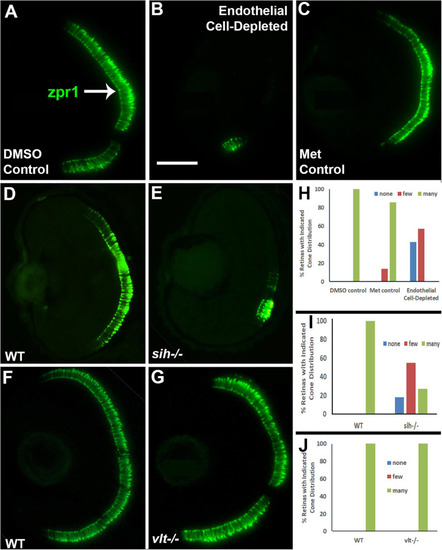
Cone photoreceptors in cardiovascular disruption model systems. |
|
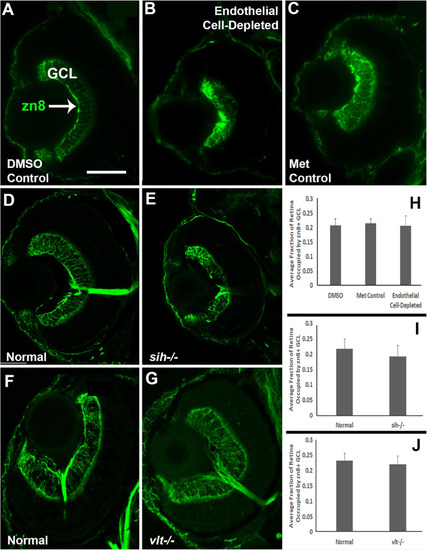
Retinal ganglion cells in cardiovascular disruption model systems. |
|
Plexiform layers in cardiovascular disruption model systems. |
|
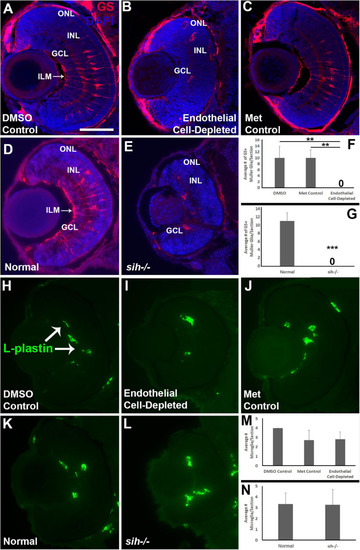
Müller glia and microglia in cardiovascular disruption model systems. (A–C) Cryosections of doubly-transgenic (cdh5:gal4; UAS:nfsB-mCherry), DMSO-treated (DMSO Control; (A); Met-treated (Endothelial Cell-Depleted; (B); and Met-treated clutchmates (Met Control; (C) at 72 hpf, stained with anti-Glutamine synthetase (GS; red fluorescence); GS is present within Müller glia, and counterstained with DAPI (blue). Control retinas show GS+ cell bodies within the INL, and GS+ Müller glial processes spanning the retina with a clear radial orientation and endfeet at the inner limiting membrane (ILM, arrow in A), while endothelial cell-depleted retinas display reduced GS staining, GS+ cell bodies in layers other than the INL, and a lack of GS+ endfeet at the ILM (B). (D,E) Cryosections of normal clutchmates (D) and sih–/– embryos (E) at 72 hpf, stained with anti-GS and DAPI. Normal retinas show normally-patterned GS+ Müller glial endfeet, cell bodies, and radial processes. The sih–/– retinas show far fewer GS+ cell bodies, and these are not positioned within the INL. (F,G) Quantification of GS+ cells with Müller glial morphology shows significantly fewer following endothelial cell depletion [(F); p < 0.01; Kruskal-Wallis with Conover post hoc test; n = 7 DMSO, 5 Met control, 6 endothelial cell-depleted], and in sih–/– compared with normal clutchmates [(G); ***p < 0.001; Mann-Whitney test, n = 8 for each condition] (H,J) Cryosections of doubly-transgenic (cdh5:gal4; UAS:nfsB-mCherry), DMSO-treated (DMSO Control; (H); Met-treated (Endothelial Cell-Depleted; I); and Met-treated clutchmates [(Met Control; (J)] at 72 hpf, stained with anti-L-plastin, which is a pan-leukocyte marker and stains microglia. Control retinas contain L-plastin+ microglia within the inner retina (H, arrows), and endothelial cell-depleted retinas also show microglia present within the inner retina (I). (K,L) Cryosections of normal clutchmates (K) and sih–/– embryos (L) at 72 hpf, stained with L-plastin, show similar patterns of staining. Scale bar (in A, applies to all images) = 50 μm. (M,N) Quantification of L-plastin+ microglia, in retinas of endothelial cell-depleted vs. control embryos [(M); p = 0.15; ANOVA with Tukey post hoc; n = 9 for each condition)], and sih–/– vs. normal siblings (N; p = 0.47; Student’s t-test; n = 7 for each condition). |
|
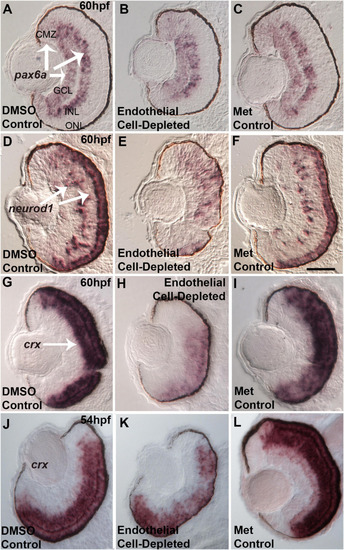
Selected retinal transcription factors in embryos depleted of vascular endothelial cells. |
|
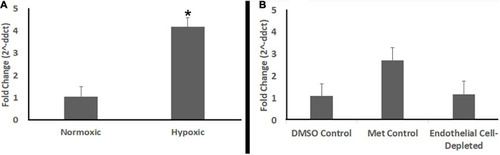
Endothelial cell-depleted embryos are not hypoxic. |