- Title
-
Zebrafish Pax1a and Pax1b are required for pharyngeal pouch morphogenesis and ceratobranchial cartilage development
- Authors
- Liu, Y.H., Lin, T.C., Hwang, S.L.
- Source
- Full text @ Mech. Dev.
|
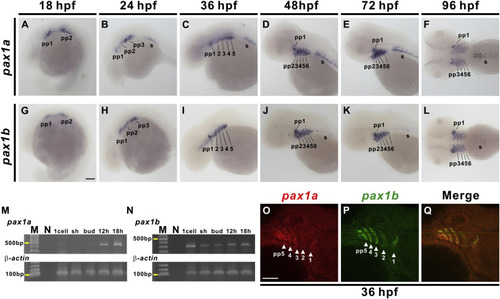
Developmental expression pattern of pax1a and pax1b. Signals for pax1a (A–F) and pax1b (G–L) were observed in the developing pharyngeal pouches (pp) and sclerotome (s) from 18 to 96 hpf. pax1a- or pax1b-hybridized embryos are shown from a lateral view at 18 hpf (A, G), 24 hpf (B, H), 36 hpf (C, I), 48 hpf (D, J) and 72 hpf (E, K), and a dorsal view is shown for 96 hpf (F, L). Semi-quantitative RT-PCR indicates expression of pax1a (M) and pax1b (N) from 1 cell to 18 hpf stages. Expression of β-actin was used as a control. Molecular weight marker (M) and no template control (N) are also shown. Double fluorescence in situ hybridization revealed co-expression of pax1a (O) and pax1b (P) in pharyngeal pouches 1–5 at 36 hpf. Merged image (Q) is shown. Lateral view of embryos is shown with anterior aspect to the right. Sh, shield. Scale bars represent 100 μm. |
|
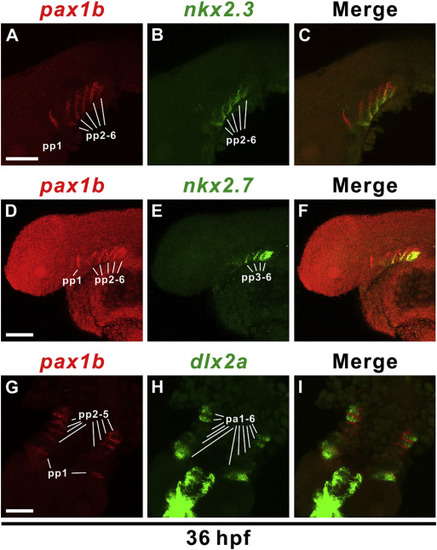
pax1b is expressed in pharyngeal pouches but not in the neural crest cells of pharyngeal arches. Double fluorescence in situ hybridization indicated pax1b (A, red) is co-expressed with nkx2.3 (B, green) in pharyngeal pouches (pp) 2–6 (C, merge) at 36 hpf. pax1b (D, red) is also co-expressed with nkx2.7 (E, green) in pharyngeal pouches 3–6 (F, merge). Expression of pax1b (G, red) in the pharyngeal pouches is adjacent to dlx2a (H, green), which is expressed in the neural crest cells of pharyngeal arches (pa). Relative localization is apparent in the merged image (I). Scale bars represent 100 μm. EXPRESSION / LABELING:
|
|
Time lapse analyses of Tg(pax1b:eGFP) enhancer trap transgenic embryos reveals sequential segmental development of pharyngeal pouches. Double fluorescence in situ hybridization indicated co-expression of egfp (A, D, green) with pax1b (B, E, red) in pharyngeal pouches (pp) 1–5 or 1–6 (C, F, merge) at 30 and 36 hpf. Time lapse analyses of Tg(pax1b:eGFP) transgenic embryos show morphogenesis of pharyngeal pouch 1–6 during 12 to 34 hpf stages. Lateral view of a Tg(pax1b:eGFP) embryo at 12 hpf (G), 12.5 hpf (H), 13 hpf (I), 14 hpf (J), 15 hpf (K), 16 hpf (L), 18 hpf (M), 20 hpf (N), 22 hpf (O), 24 hpf (P), 26 hpf (Q), 28 hpf (R), 30 hpf (S), 32 hpf (T) and 34 hpf (U) are shown. Scale bars represent 100 μm. EXPRESSION / LABELING:
|
|
Pax1a and Pax1b have redundant function in regulating pharyngeal cartilage development. Mandibular pharyngeal arch 1 (Meckel's cartilage (m) and palatoquadrate (pq)), hyoid pharyngeal arch 2 (hyosymplectic (hs), interhyal (ih), ceratohyal (ch)) and five branchial arches (ceratobranchial (cb), hypobranchial (hb) and basibranchial (bb)) were identified in Alcian blue-stained wild types (A) and embryos injected with 0.75 ng each of 5 mm pax1a MO and 5 mm pax1b MO (B) at 96 hpf are shown. Mild pharyngeal cartilage defect (C, shortened cb 2–3, lack of cb 1 and 4 on one side and lack of cb1 on the other side) and two types of severe cartilage defects (D, straightened ch, shortened cb 2–3, lack of cb 1 and 4 on one side and lack of cb1–4 on the other side; E, reversed ch and lack of cb 1–4 on both sides) were detected in respective embryos injected with 1.5 ng pax1a MO (MOa), 10 ng pax1b MO (MOb), or 0.75 ng of both pax1a MO and pax1b MO. The majority of embryos injected with suboptimal dose (0.75 ng) of both pax1a MO and pax1b MO exhibited severe pharyngeal cartilage defects, while embryos injected with 1.5 ng pax1a MO or 10 ng pax1b MO showed less frequent severe phenotypes (F, G). Number of embryos for various treatment is shown under the x-axis. Brackets indicate lack of cb1–4. Scale bar represents 100 μm. PHENOTYPE:
|
|
pax1a- and pax1b-deficient embryos generated by CRISPR-Cas9 mutagenesis exhibit abnormality of hyoid cartilage and lack of ceratobranchial cartilages 1–4. Genomic structures of pax1a (A) and pax1b (B) include the target sites for pax1a sgRNA and pax1b sgRNA in exon 2 of each gene. Nucleotide sequence of wild type (WT) and sequences of pax1aas36 and pax1bas37 mutants containing 5-bp deletions (red dashed line) are shown (A, B). sgRNA sequence (yellow highlight) and protospacer adjacent motif (PAM, blue highlight) are indicated. (C, D) Sequences of WT and pax1aas36 or pax1bas37 mutants were compared. Positions of deleted sequences in pax1aas36 or pax1bas37 mutants are indicated by arrowheads. Normal patterning of pharyngeal arches was detected in Alcian blue-stained uninjected (E) or 1.5 ng pax1b MO-injected pax1a+/− incrossed embryos (F) at 96 hpf. About 21.1% of pax1a+/− incrossed embryos that had been injected with 1.5 ng pax1b MO failed to develop ceratobranchial cartilages (G, H). Similarly, normal pharyngeal cartilage development was identified in Alcian blue-stained uninjected pax1b−/− incrossed embryos (I) while loss of ceratobranchial cartilage and hyoid cartilage with inverted (J) or no (K) anterior-posterior polarity were detected in 0.75 ng pax1a MO-injected pax1b−/− incrossed embryos stained with Alcian blue at 96 hpf. A comparison of the percentage embryos with pharyngeal cartilage defects in uninjected and pax1a MO-injected pax1b−/− incross embryos is shown (L). A pax1a−/−; pax1b−/− double mutant embryo (N) exhibits a straight body, lack of swim bladder (arrowhead) and lack of gill cartilages (arrow), in contrast with wild type (M) at 96 hpf. Alcian blue-stained pax1a−/−; pax1b−/− double mutant embryo (P) has no ceratobranchial cartilage, in contrast with pax1a−/−; pax1b+/− mutant embryo (O) with normal pharyngeal cartilages at 96 hpf. Flat mount Alcian blue staining of pharyngeal cartilage revealed loss of ceratobranchial (cb) cartilages 1–4 with retention of shorter cb cartilage 5 in pax1a−/−; pax1b−/− double mutants. Basibranchial (bb) cartilages 2–5 were also absent, with retention of bb cartilage 1. All hypobranchial (hb) cartilages and anterior hyomandibula (hm, arrowhead) were also absent in pax1a−/−; pax1b−/− double mutants (S) in contrast to wild types (Q) and pax1b−/− mutant embryos (R) at 96 hpf. Brackets indicate lack of cb1–4. Ch, ceratohyal; ih, interhyal; m, Meckel's cartilage; op, opercular bone; pq, palatoquadrate; sy, symplectic; te, teeth. Scale bars represent 100 μm. PHENOTYPE:
|
|
pax1a- and pax1b-deficient embryos display decreased or absent dlx2a and hand2 expression in pharyngeal arches. Reduced expression of dlx2a (D) or hand2 (I) as well as absence of dlx2a (E) or hand2 (J) in the posterior pharyngeal arches (pa) was identified in 0.75 ng pax1a MO-injected pax1b−/− mutant embryos compared to uninjected pax1b−/− mutant (C, H), wild types (WT; A, F) or 0.75 ng pax1a MO-injected wild types (B, G) at 36 hpf. Similar defects in expression of dlx2a (L) or hand2 (N) in the posterior pharyngeal arches were also identified in pax1a−/−; pax1b−/− double mutants compared with sibling controls (i.e., pax1a+/−; pax1b−/− or pax1a+/+; pax1b−/−) (K, M) at 36 hpf. Asterisks indicate reduced or absent expression. Affected embryos per total number of analyzed embryos are shown. Scale bars represent 100 μm. |
|
Reduced dlx2a expression in pharyngeal arches 3–4 in pax1a; pax1b morphants beginning at 22 hpf. Similar dlx2a expression levels were detected in three migrating streams (S1, S2, S3) of cranial neural crest cells in uninjected (C), 0.75 ng pax1a MO-injected pax1b−/− mutant embryos (D), uninjected wild types (WT; A) or 0.75 ng pax1a MO-injected wild types (B) during 18–22 s stages. Similar dlx2a expression levels in three migrating streams of cranial neural crest cells were observed in wild types and pax1a; pax1b morphants at 18 hpf (E, L) and 20 hpf (F, M). However, reduced dlx2a expression (asterisk) was identified in pharyngeal arches (pa) 3–4 of pax1a; pax1b morphants compared to wild-type embryos at 22 hpf (G, N), 24 hpf (H, O), 26 hpf (I, P), 28 hpf (J, Q) and 30 hpf (K, R). Scale bars represent 100 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
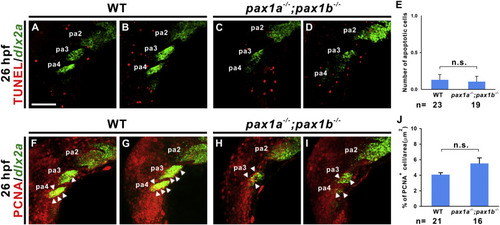
Similar levels of apoptosis and proliferation were identified in pharyngeal arches 3–4 of wild types and pax1a- and pax1b-deficienct embryos. TUNEL assay was performed in wild types (WT; A, B) and pax1a−/−; pax1b−/− double mutant (C, D) embryos that were hybridized with dlx2a antisense RNA at 26 hpf. Quantification of apoptotic cells in pharyngeal arches (pa) 3 and 4 in wild types and mutants is shown (E). Proliferation was evaluated by anti-PCNA antibody in wild types (F, G) and pax1a−/−; pax1b−/− double mutant (H, I) embryos that were hybridized with dlx2a antisense RNA at 26 hpf. Quantification of proliferation rate in pharyngeal arches 3 and 4 in wild types and mutants is shown (J). Arrowheads indicate neural crest cells labeled with PCNA and dlx2a. Statistical significance was evaluated by Student's t-test, NS, not significant. Error bars indicate standard error. Scale bar represents 100 μm. PHENOTYPE:
|
|
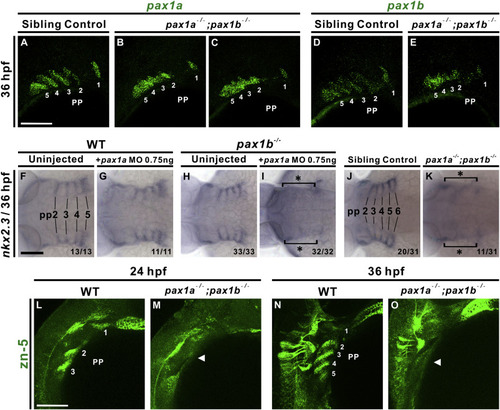
pax1a- and pax1b-deficient embryos exhibit morphogenetic defects and lack of nkx2.3 or Alcama expression in pharyngeal pouches. Segmented pharyngeal pouch (pp) 1 expresses pax1a or pax1b, while unsegmented pharyngeal pouches 2–5 display continuous expression of pax1a or pax1b and small outpocketings in pax1a−/−; pax1b−/− double mutant embryos (B, C, E); sibling controls (i.e., pax1a+/−; pax1b−/− or pax1a+/+; pax1b−/−) show normal segmentation (A, D) when hybridized with pax1a or pax1b RNA probes at 36 hpf. Absent expression of nkx2.3 in the pharyngeal pouches (pp) 2–5 was observed in 0.75 ng pax1a MO-injected pax1b−/− mutant embryos (I) compared to un-injected pax1b−/− mutants (H), wild types (WT; F) or 0.75 ng pax1a MO-injected wild types (G) at 36 hpf. A similar defect in nkx2.3 expression in pharyngeal pouches 2–6 was also identified in pax1a−/−; pax1b−/− double mutants (K) compared with sibling controls (i.e., pax1a+/−; pax1b−/− or pax1a+/+; pax1b−/−) (J). A lack of Alcama expression (arrowhead; labeled by anti-zn5 antibody) was noted in the pharyngeal pouches of pax1a−/−; pax1b−/− double mutants in contrast with wild types at 24 hpf (L, M) and 36 hpf (N, O). Scale bars represent 100 μm. |
|
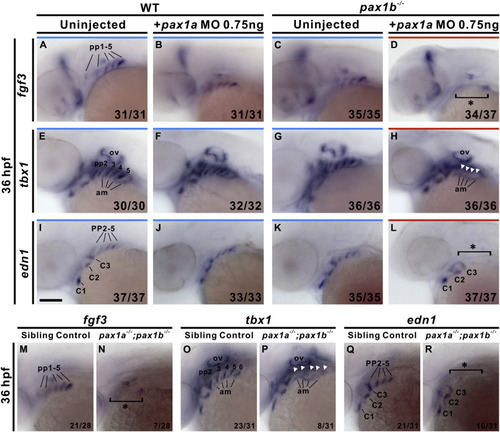
pax1a- and pax1b-deficient embryos exhibit absent fgf3, tbx1 and edn1 expression in pharyngeal pouches. Absent expression of fgf3 in pharyngeal pouches (pp) 1–5 was identified in 0.75 ng pax1a MO-injected pax1b−/− mutant embryos (D) compared to uninjected wild types (WT; A), 0.75 ng pax1a MO-injected wild types (B) or uninjected pax1b−/− mutant embryos (C) at 36 hpf. Similar absent expression of tbx1 or edn1 in posterior pharyngeal pouches 2–5 were observed in 0.75 ng pax1a MO-injected pax1b−/− mutant embryos (H, L) compared to uninjected wild types (E, I), 0.75 ng pax1a MO-injected wild types (F, J) or uninjected pax1b−/− mutant embryos (G, K) at 36 hpf. Similar defects in endodermal expression of fgf3 (N), tbx1 (P) and edn1 (R) in pharyngeal pouches were also found in pax1a−/−; pax1b−/− double mutants in contrast with sibling controls (i.e., pax1a+/−; pax1b−/− or pax1a+/+; pax1b−/−) (M, O, Q) at 36 hpf. Asterisks or arrowheads indicate absent expression. Affected embryos per total number of analyzed embryos are shown. Am, adjacent mesoderm; C, mesodermal core; ov, otic vesicle. Scale bars represent 100 μm. |
Reprinted from Mechanisms of Development, 161, Liu, Y.H., Lin, T.C., Hwang, S.L., Zebrafish Pax1a and Pax1b are required for pharyngeal pouch morphogenesis and ceratobranchial cartilage development, 103598, Copyright (2020) with permission from Elsevier. Full text @ Mech. Dev.