- Title
-
Genetic dissection of endothelial transcriptional activity of zebrafish aryl hydrocarbon receptors (AHRs)
- Authors
- Sugden, W.W., Leonardo-Mendonça, R.C., Acuña-Castroviejo, D., Siekmann, A.F.
- Source
- Full text @ PLoS One
|
Zebrafish AHR genes are differentially expressed during development. (A) Schematic of genomic arrangement of zebrafish AHR genes. Note linkage of ahr1b and ahr2 (~3 kb intergenic distance). (B) Experimental design. Embryos were exposed to DMSO or 1 uM BNF for 4 hrs starting at 48 hpf, then fixed in 4% PFA for ISH. (C-H) Stereomicroscope images of whole mount ISH in 52 hpf embryos exposed to DMSO or 1 uM BNF showing expression of ahr1a (C, D), ahr1b (E, F) and ahr2 (G, H). Boxes indicate region of high magnification image in inset. Numbers indicate embryos with indicated expression pattern/total embryos analyzed. Scale bar is 500 um. (I-N) High magnification images of trunk in 52 hpf embryos exposed to DMSO or 1 uM BNF showing expression of ahr1a (I, J), ahr1b (K, L) and ahr2 (M, N). No staining evident for ahr1a or ahr1b in either condition. ahr2 expression is detected in DLAV, gut (white arrowhead) and region between DA and PCV (white bracket), and is increased by BNF-treatment. Scale bar is 100 um. Abbreviations—AHR: aryl hydrocarbon receptor, BNF: beta-naphthoflavone, DA: dorsal aorta, DLAV: dorsal longitudinal anastomotic vessel, DMSO: dimethylsulfoxide, hpf: hours post fertilization, ISH: in situ hybridization, kb: kilobase pair PCV: posterior cardinal vein, PFA: paraformaldehyde. |
|
Differential vascular expression of CYP genes under basal and BNF-induced conditions. (A) Stereomicroscope images of whole mount ISH for cyp1a1 and cyp1b1 in 52 hpf WT embryos after 4 hrs exposure to DMSO (A) or 1 uM BNF (B). Strong upregulation of cyp1a1 in skin can be detected. cyp1b1 is also induced in the skin, but to a lesser extent. Numbers indicate embryos with indicated expression pattern/total embryos analyzed. Scale bar is 500 um. (C). Camera lucida drawing of 48 hpf zebrafish embryo from Kimmel, et al 1995 [36]. Dashed lines indicate plane of images to visualize central arteries (CtAs, red) and hindbrain vessels (blue). Confocal image shows dorsal view of hindbrain vasculature of live embryo at 48 hpf (scale bar is 200 um). Cartoon schematic depicts arrangement of hindbrain vessels. (D-I) High magnification images of basal cyp1a1/b1 expression at 52 hpf in the hindbrain (D, G) and CtAs (E, H) of the head vasculature in embryos treated with DMSO. Schematics in F,I summarize hindbrain expression patterns of both genes. (J-O) High magnification images of BNF-induced cyp1a1/b1 expression at 52 hpf in the hindbrain (J, M) and CtAs (K, N) of the head vasculature in embryos treated with 1 uM BNF. Schematics in L,O summarize hindbrain expression patterns of both genes. Analysis of cyp1a1 expression was performed in ahr2 mutant embryos to permit visualization of hindbrain vasculature. Yellow arrowheads mark CtAs. Scale bar is 100 um in all high magnification images. Abbreviations–AHR: aryl hydrocarbon receptor, BA: basilar artery, BCA: basal communicating artery, BNF: beta-naphthoflavone, CtA: central artery, CYP: cytochrome p450, DMSO: dimethylsulfoxide, hpf: hours post fertilization, ISH: in situ hybridization, PCS: posterior communicating segments, PHBC: primordial hindbrain channel, WT: wildtype. |
|
Generation of zebrafish ahr1a and ahr1b mutants. (A, B) Depiction of genomic locus for ahr1a (A) and ahr1b (B). TALEN pairs were designed targeting the bHLH domain in exon 2 of both genes. Genomic lesions in the TALEN spacer indicated in alignment to WT sequence, and cartoons show predicted protein products. For ahr1b, alleles mu133-mu135 were generated in a WT genetic background, while mu136-mu145 were made in an ahr2hu3335 homozygous mutant background. (C) Brightfield images of WT, ahr1a -/-, ahr1b,2 -/- and triple AHR mutant embryos at 5 dpf. No gross morphological defects in AHR mutants are apparent. Boxes indicate region of imaging for different vascular beds in D-I. Numbers correspond to “number of embryos with inflated swim bladders/total number of embryos of that genotype”. Scale bar is 1000 um. (D-I) Maximum intensity projections of confocal z-stacks comparing the trunk (D, E), brain (F, G) and liver (H, I) vascular beds in ahr1a -/- and triple AHR mutant zebrafish embryos at 5 dpf. Vascular patterning appears normal in both mutants compared to WT. Yellow arrowheads point to forming intercostal vessels in the trunk. Orange brackets mark midbrain vessels, yellow brackets mark hindbrain vessels. Red arrows show the liver. Numbers indicate the “number of embryos of a particular genotype with vascular characteristics as in image/total numbers of that genotype analyzed.” Scale bar is 100 um. Abbreviations—AHR: aryl hydrocarbon receptor, bHLH: basic helix-loop-helix, hpf: hours post fertilization, TALEN: transcription activator-like effector nuclease, WT: wildtype. |
|
Regulation of cyp1a1 by AHR. (A) Overview pictures of cyp1a1 expression at 52 hpf in DMSO or BNF-treated WT (A), ahr1a -/- (B), ahr2 -/- (C), ahr1b,2 -/- (D) and triple AHR mutants (E). ahr1a -/- are indistinguishable from WT. (*) indicates expression in the eye in DMSO and BNF-treated embryos, which is lost only in ahr1b,2 -/- and triple AHR mutants. Note reduction of endogenous vascular cyp1a1 expression in ahr2 mutants (C, upper panels) that is lost in ahr1b,2 -/- and triple AHR mutants (D, E upper panels). ahr2 -/- embryos treated with BNF do not induce cyp1a1 expression in the skin, revealing staining in blood vessels underneath (C, lower panel), which is abolished in ahr1b,2 -/- and triple AHR mutants (D, E, lower panels). Scale bar is 500 um. (F-T) High magnification images of blood vessels of 3 different planes of the head vasculature: the LDA (F-J), the hindbrain blood vessels (K-O) and the hindbrain capillaries (P-T) in all genetic combinations with DMSO and BNF treatment. Note strong dependence of LDA (but not hindbrain arteries, PHBC or CtAs) on ahr2 for full cyp1a1 expression. White asterisk denotes liver. Yellow arrowheads label individual CtAs. Numbers indicate embryos with indicated expression pattern/total embryos of that genotype analyzed. Scale bar is 100 um. Abbreviations–AHR: aryl hydrocarbon receptor, BNF: beta-naphthoflavone, CtA: central artery, CYP: cytochrome p450, DMSO: dimethylsulfoxide, hpf: hours post fertilization, LDA: lateral dorsal aortae, PHBC: primordial hindbrain channel, WT: wildtype. |
|
Regulation of cyp1b1 by AHR. (A-E) Overview pictures of cyp1b1 expression at 52 hpf in DMSO or BNF-treated WT (A), ahr1a -/- (B), ahr2 -/- (C), ahr1b,2 -/- (D) and triple AHR mutants (E). No changes in expression pattern were evident in DMSO-treated embryos that lacked any AHR genes. Induction in the skin by BNF was dramatically reduced in ahr2 -/- (compare A to C, lower panels). Scale bar is 500 um. F-T) High magnification images of blood vessels in 3 different planes of the head vasculature: the LDA (F-J), the hindbrain blood vessels (K-O) and the hindbrain capillaries (P-T) in all genetic combinations with DMSO and BNF treatment. Black asterisks denote approximate location of liver. Yellow arrowheads label individual CtAs. Note maintenance of endogenous cyp1b1 expression in the ear (K-O, upper panels) and head (P-T, upper panels) of all mutants. Expression of cyp1b1 was not detected in the liver for either treatment in any genetic combination (F-J). Note lack of BNF-induced staining in LDA of ahr2 mutants, but strong staining in PHBCs (H and M, lower panels). Numbers indicate embryos with indicated expression pattern/total embryos of that genotype analyzed. Scale bar is 100 um. Abbreviations–AHR: aryl hydrocarbon receptor, BNF: beta-naphthoflavone, CtA: central artery, CYP: cytochrome p450, DMSO: dimethylsulfoxide, hpf: hours post fertilization, LDA: lateral dorsal aortae, PHBC: primordial hindbrain channel, WT: wildtype. |
|
Transcriptional control of cxcr4a by AHR. (A) Core DNA binding sequence of AHR::ARNT complex from JASPAR database. (B) Schematic of the zebrafish cxcr4a gene. Using strict search criteria (a relative profile score threshold of 90%), the promoter sequence spanning 2 kb from the start codon was analyzed for AHR binding sites in the JASPAR database. Two putative AHR binding sites lie close to the transcription start site (TSS). (C-H) Whole mount ISH for cxcr4a in WT and triple AHR mutant embryos treated from 48 hpf to 52 hpf with DMSO (C, F), 1 uM BNF (D, G) or 15 uM nifedipine (E, H). Arrows indicate subintestinal vasculature, while arrowheads point to DLAV. Scale bar is 250 um. (I-K) High magnification images of hindbrain capillaries in WTs and triple AHR mutants. Note similar levels of cxcr4a-positive CtAs (yellow arrowheads) in WTs and triple AHR mutants treated with DMSO (I), and markedly fewer cxcr4a-positive cells in triple mutants treated with BNF compared to WT (J). Both WTs and triple AHR mutants display marked increase in cxcr4a expression in all CtA endothelial cells after nifedipine treatment (K). Scale bar is 100 um. (L) Quantification of cxcr4a-positive endothelial cells in high magnification images of the hindbrain of WT, ahr1a -/-, ahr1b,2 -/- and triple AHR mutants treated from 48–52 hpf with DMSO or nifedipine. All genetic combinations of AHR mutations have similar numbers of cxcr4a-positive cells at basal levels. The BNF-induced increase of cxcr4a expression in WT still occurs in ahr1a -/- embryos, but does not show in ahr1b,2 -/- or triple AHR mutants. Analyzed by One-way ANOVA (DMSO treatment N = 4 WT, 12 ahr1a -/-, 13 ahr1b,2 -/- and 14 triple AHR fish. 1 uM BNF treatment N = 9 WT, 12 ahr1a -/-, 10 ahr1b,2 -/- and 6 triple AHR fish.) (M) Summary of transcriptional control of cxcr4a expression by flow (negative regulator) and BNF (positive regulator). Induction by BNF is in an AHR-dependent manner. **P<0.01, error bars indicate s.e.m. Abbreviations–AHR: aryl hydrocarbon receptor, ARNT: aryl hydrocarbon nuclear translocator, BNF: beta-naphthoflavone, CtA: central artery, CYP: cytochrome p450, DLAV: dorsal longitudinal anastomotic vessel, DMSO: dimethylsulfoxide, hpf: hours post fertilization, ISH: in situ hybridization, LDA: lateral dorsal aortae, PHBC: primordial hindbrain channel, TSS: transcription start site, WT: wildtype. |
|
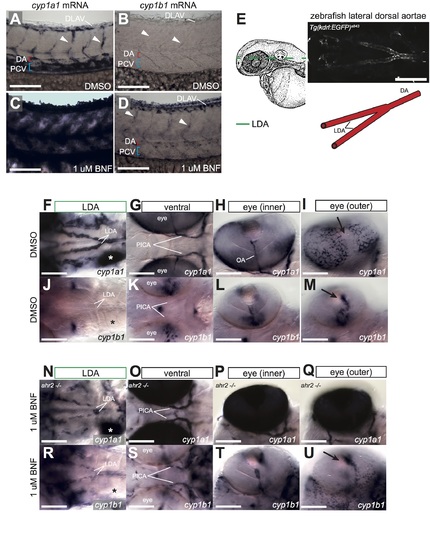
Endogenous and BNF-induced CYP1 expression in other vascular beds. A-D) High magnification images of cyp1a1/b1 expression in trunk vasculature of DMSO (A, B) and BNF (C, D) treated embryos. Arrowheads point to ISVs. cyp1a1 is expressed highly in PCV, ISVs and DLAV, and in isolated DA cells under normal conditions (A), and possible induction in blood vessels by BNF is obscured by high skin staining (C). No endogenous vascular cyp1b1 can be detected (B), but it is induced in DA, DLAV and ISVs by BNF (D). E) Camera lucida drawing of 48 hpf zebrafish embryo from Kimmel, et al 1995 [36]. Dashed lines indicate plane of images in this figure to visualize lateral dorsal aortae (LDA, green). Confocal image shows dorsal view of LDA in live embryo at 48 hpf (scale bar is 200 um). Cartoon schematic depicts arrangement of LDA and DA. F-M) Cranial expression of cyp1a1/b1 in DMSO-treated embryos. Expression of cyp1a1 is mostly vascular-specific and restricted to arteries (LDA, PICA and OA). (*) marks the liver. No vascular or liver expression of cyp1b1 is detected, and staining is only evident in the ear, middle of the brain and parts of the ventral eye. Arrow marks optic furrow. N-U) Cranial expression of cyp1a1/b1 in BNF-treated embryos. In addition to the basal expression domains, specific blood vessels upregulate cyp1b1 (the LDA and PICA). Note lack of cyp1b1 expression in the liver even under BNF stimulation (* in R). Embryos in N-Q are ahr2 mutants to enable imaging of interior vessels. Numbers of embryos analyzed are the same as in Fig 2. All scale bars are 100 um. Abbreviations–AHR: aryl hydrocarbon receptor, BNF: beta-naphthoflavone, CYP: cytochrome p450, DA: dorsal aorta, DLAV: dorsal longitudinal anastomotic vessel, DMSO: dimethylsulfoxide, hpf: hours post fertilization, ISV: intersegmental vessel, LDA: lateral dorsal aortae, OA: optic artery, PCV: posterior cardinal vein, PICA: primitive internal carotid artery. |
|
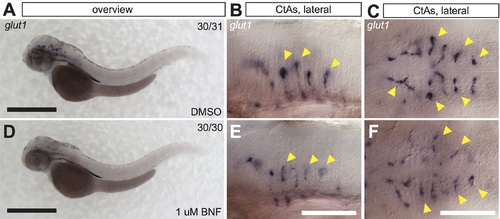
Vascular glut1 expression in WT embryos with and without BNF treatment. A-C) Overview and high magnification images of whole mount ISH for glut1 in WT embryos at 52 hpf. Vascular expression is limited to the brain capillaries (yellow arrowheads indicate individual CtAs). D-F) Glut1 expression in WT embryos treated with 1 uM BNF. Staining intensity is notably decreased in brain vessels. Scale bar in overview is 500 um, and 100 um in high magnification images. Abbreviations–BNF: beta-naphthoflavone, CtA: central artery, DMSO: dimethylsulfoxide, hpf: hours post fertilization, ISH: in situ hybridization. |
|
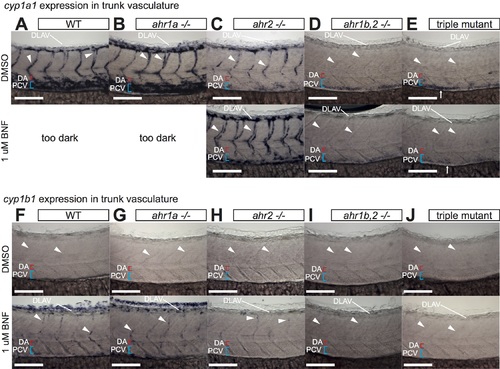
Analysis of vascular CYP1 expression in trunk of AHR mutants. A-J) High magnification images of cyp1a1/b1 expression in the trunk vasculature at 52 hpf in DMSO or BNF-treated WT (A, F), ahr1a -/- (B, G), ahr2 -/- (C, H), ahr1b,2 -/- (D, I) and triple AHR mutants (E, J). Expression patterns of both genes in ahr1a -/- are indistinguishable from WT in either condition. In ahr2 mutants a dramatic loss of endogenous cyp1a1 expression from the PCV and DA is observed, together with a weaker but persistent expression in DLAV and ISVs that is enhanced by BNF treatment (C). This remaining vascular expression is lost in ahr1b,2 -/- and triple AHR mutants (D, E). Note the AHR-independent expression of cyp1a1 in the gut (arrows in E) Similar results are seen in the BNF-induced cyp1b1 expression, which is weakly maintained in ISVs and DLAV of ahr2 mutants and lost in ahr1b,2 -/- and triple AHR mutant embryos (F-J). Numbers of embryos analyzed are the same as in Fig 4 (cyp1a1) and Fig 5 (cyp1b1). All scale bars are 100 um. Abbreviations–AHR: aryl hydrocarbon receptor, BNF: beta-naphthoflavone, CYP: cytochrome p450, DA: dorsal aorta, DLAV: dorsal longitudinal anastomotic vessel, DMSO: dimethylsulfoxide, hpf: hours post fertilization, ISV: intersegmental vessel, PCV: posterior cardinal vein, WT: wildtype. |