- Title
-
Cited3 activates Mef2c to control muscle cell differentiation and survival
- Authors
- Devakanmalai, G.S., Zumrut, H.E., and Ozbudak, E.M.
- Source
- Full text @ Biol. Open
|
Expression of cited3 during segmentation stage. (A) cited3 is expressed in the polster (black arrow) and in the notochord (red arrow) and chordo-neural hinge (green arrowhead) at 10 hpf. (B) At 13 hpf, cited3 is expressed in the adaxial cells (slow muscle precursor; black arrowhead) on either side of the notochord. (C) Expression of cited3 becomes abundant in the slow muscle precursors at 14 hpf. (D) cited3 is also expressed in the differentiated slow muscle cells as indicated by black arrowhead and in the hatching gland as indicated by black arrow and neural crest cells (red arrowhead) at 24 hpf. (E–H) cited3 is expressed in the differentiating slow muscle cells. (E) Cross section of 24 hpf embryo depicting Hoechst staining in the nucleus; (F) cited3 expression in the slow muscle domain; (G) myod expression in the slow and fast muscle domain; (H) merge of cited3 and myod expression in the slow muscle domain (indicated by a white arrowhead). (I–K) cited3 is expressed in the heart precursors. (I) cited3 is expressed in the heart precursors both in the atrium and ventricle (J) cmlc2 expression in the heart precursors (K) merge (ventral view of embryos). (L–S) Expression of cited3 is regulated by the Hedgehog signaling (L,N) Expression of myoD, cited3 was not perturbed in ethanol treated control embryos, (M,O) but expression of myod and cited3 is lost in the adaxial cells in cyclopamine treated (from 5–15hpf) embryos (indicated by arrowhead) whereas myod expression is still retained in the fast muscle (M) and cited3 expression in the chordo-neural-hinge (arrow) (O). In comparison to control ethanol treatment, cyclopamine treatment from 13 to 18 hpf blocked transcription of myod in all adaxial cells (P,Q), but that of cited3 only in adaxial cells located in the presomitic mesoderm (R,S). (T–W) Myod and Myf5 redundantly regulate the expression of cited3. (U) Expression of cited3 was not perturbed in the embryos that were injected with myoD MO compared to those injected with the control MO (T) at 17 hpf. Similarly, expression of cited3 was not affected in 75% of the embryos that were obtained from mating of heterozygous myf5hu202 mutant fishes and injected with the myod MO (22/28) (V,V′). However, expression of cited3 was reduced or lost in the remaining 25% of the embryos from the same clutch injected with myod MO (6/28) (W,W′), while the remaining expression of cited3 is in the neural crest cells. Scale bars: 100μm in D,L,P,T, and 50μm in V′. |
|
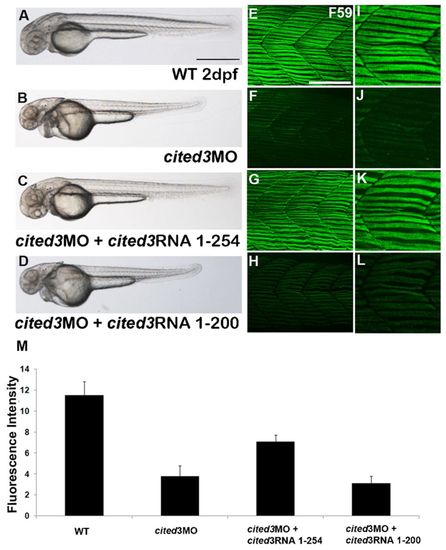
Knock down of cited3 disrupts muscle differentiation. (A,B) Knock down of cited3 with two splice-blocking morpholino oligonucleotides (MO), S1-MO and S2-MO, resulted in thinner and shorter trunk, and dilated heart (B) compared to wild-type embryos (A) or embryos injected with the control MO (data not shown). Coinjection of full length cited3 RNA (codes for 254 amino acid) rescued cited3 morphant phenotype (C). (D) In contrast, coinjection of a C-terminally truncated form of cited3 RNA (coding for the first 200 amino acids) did not rescue the morphant phenotype. (E) The expression of myosin heavy chain as evidenced by F59 antibody in an area covering somite 16th–18th in a wild-type embryo at 2 dpf. (F) The staining of F59 is reduced in embryos injected with cited3 MO, whereas the expression of myosin heavy chain was restored when cited3 RNA was co-injected with cited3 MO (G) but not rescued when cited31–200 RNA was coinjected (H). (I–L) Magnified images of F59 staining. (M) The graphical representation of the average fluorescent intensity measurement of F59 immunostaining (of 10–18 embryos from three different experiments). Intensity of F59 staining was significantly reduced in cited3 morphants when compared to that in wild-type (P-value<2×104), rescued when cited3 RNA was co-injected (P-value<2×102) but not rescued when cited31–200 RNA was coinjected (P-value>0.7). Scale bars: 100 μm in A and 50 μm in E. |
|
Knockdown of cited3 results in increased cell death but it does not affect proliferation. Embryos that are injected with the control MO (A–C), cited3 MO (D–F), cited3 MO + cited3 RNA (G–I) were fixed at 2 dpf and immunostained with anti-Mef2 antibody (A,D,G) and TMR red in situ cell death detection kit (B,E,H). Tunel positive cells were counted in non-muscle cells as well as in muscle cells in each group of embryos from 50–100 sections obtained from trunk and tail somites. More apoptotic muscle cells were detected in embryos that are injected with the cited3 MO and fixed at 2 dpf and 3 dpf (M). Values were normalized compared to cited3 morphant values. Similarly, embryos injected with the control MO (J), cited3 MO (K), cited3 MO + cited3 RNA (L) was pulsed with BrdU from 30 hpf to 42 hpf, and were fixed and immunostained with anti-BrdU and MF20 antibodies (images of 16th–18th somites). There is no significant difference between the three treated groups (J,K,L) when the BrdU-positive muscle cells were counted (data not shown). Scale bars: 25 μm in A and 50 μm in K. EXPRESSION / LABELING:
PHENOTYPE:
|
|
The number of slow myofibers is reduced in cited3 morphants starting at 3 dpf. The number of slow fibers was counted on whole mount embryos on one hemisegment from 10th-12th somites in the anterior region and 16th-18th somite in the posterior region of embryos at 1-4 dpf. Slow myofiber number is reduced significantly starting from 3 dpf in the morphant compared to WT (P-value<2×10-2 at 3 dpf and P-value<2×10-3 at 4 dpf) and was restored when cited3RNA was coinjected (P-value<4×10-2 at 3 dpf and P-value<5×10-3 at 4 dpf). PHENOTYPE:
|
|
Cited3 regulates expression of mef2c and myogenin and it acts cell-autonomously. (A–D) Expression of myoD and myf5 was not perturbed in embryos injected with the cited3 MO at 19 hpf. (E,F) Myogenin transcripts were retained in mature somites in the cited3 morphants at 22 hpf (F) where it was usually downregulated in the control MO-injected embryos (E). Transcription of mef2ca was reduced in cited3 morphants (H) compared to control MO-injected embryos (G) and it was restored to normal levels when coinjected with cited3 RNA (I) at 19 hpf. (J–L) Mef2d expression was not affected in embryos injected with the cited3 MO or cited3 MO + cited3 RNA at 19 hpf. (M–O) Immunostaining with the general anti-Mef2 antibody (recognizes all Mef2 variants) revealed that cumulative Mef2 protein levels were reduced in the cited3 morphants (N compared to M) and were restored to normal levels when cited3 RNA was coinjected (O) at 22 hpf (images spanning somites 16th–18th are shown) . Similarly, Mef2 protein levels were reduced in the heart of cited3 morphants (N′) compared to the control MO-injected embryo (M′) and rescued in the cited3 RNA coinjected embryos (O′) at 30 hpf. (P–U′) Slow muscle fibers labeled with rhodamine dextran dye were transplanted from wild-type donor embryos into control MO-injected (P–R′) or cited3 MO-injected host embryos (S–U′). Anti-Mef2 immunostaining revealed that Mef2 protein levels in wild-type transplanted myofibers (arrowhead) were not different from those in the control MO injected host myofibers (Q), but were brighter compared to those in the cited3 MO containing host slow myofibers (T). (V–X2) Mef2 staining in the transplanted cited3 morphant myofibers is fainter than their wild-type host counterparts. (Y,Z) The normalized intensity of Mef2 protein level is plotted for different transplantation conditions. The number of quantified transplanted slow myofibers ranged from 23 to 141. P-values are 107 in Y and 1012 in Z. Scale bars: 100μm in A and 25μm in M,P. Images in (P–U′) are from trunk somites, while those in (V–X′) are from anterior somites. EXPRESSION / LABELING:
|
|
Expression of various muscle structural genes were affected in cited3 morphants starting at 2 dpf. As differentiation proceeds, the expression levels of smyhc, stnnc, tnnt1, myhz2, mylc2, tnnt3b and myhz1 in wild-type embryo were down regulated at 2 dpf (A,C,E,G,I,K,M). However, their respective expression in cited3 morphants were retained (B,D,F,H,J,L,N). Expression of fast muscle myosin heavy chain is reduced after 2 dpf in cited3 morphants (P) compared to control MO injected embryos (O) as evidenced by F310 immunostaining (somites 15th–18th). Scale bars: 100 μm in B and 25 μm in P. EXPRESSION / LABELING:
|
|
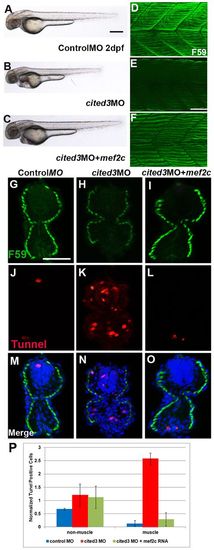
Restoring expression of Mef2c rescues functional loss of cited3 and prevents cell death. Coinjection of mouse Mef2c RNA rescues the morphological phenotypes (A–C) and the reduction in the expression of myosin heavy chain (15th–18th somites) (D–F) in the cited3 MO-injected embryos. Coinjection of mouse Mef2c RNA also reduced the number of apoptotic muscle cells in cited3 MO-injected embryos. (H,K,N) The number of apoptotic muscle cells are increased in cited3 MO-injected embryos and it was restored to normal levels by the coinjection of mouse Mef2c RNA (as seen in I,L,O) as compared to control MO injected embryos (G,J,M). (P) Quantification of the apoptotic cells revealed that mouse Mef2c RNA significantly prevented the cell death in muscle cells of embryos that are coinjected with cited3 MO. These are average values from 97 sections from trunk and tail of 5–10 embryos from three different experiments. Values were normalized compared to cited3 morphant values. Scale bars: 100 μm in A and 25 μm in E,G. |
|
WNT and FGF signaling do not regulate expression of cited3. We utilized the hsp70: Δtcf-gfp transgenic line to block Wnt signaling by expression of dominant-negative tcf (A,B), the hsp70:wnt8-gfp transgenic line to overactivate Wnt signaling by expression of wnt8 (C,D) and the hsp70:dnfgfr1 transgenic line to block Fgf signaling by expression of dominant negative fgfr1 (E,F). Embryos were collected by mating heterozygous transgenic animals. At 15 hpf, embryos were heatshocked for 1 hour at 37°C and were revived for 2 h at 28°C. As expected 25% of the embryos (wild-type control siblings) did not express the transgene (A,C,E), while the remaining 75% of the embryos expressed the respective transgene (B,D,F). Expression of cited3 was not affected when WNT signaling was blocked (A,B), or over activated (C,D). Expression of cited3 was also not disturbed when FGF signaling was blocked (E,F) at 18 hpf. |
|
Knockdown of cited3 leads to muscle growth defects and reduction in myofiber number. (A,B) Slow fiber numbers are counted by using the slow fiber specific antibody S58 in the anterior somites (10th–12th). Fiber numbers, fiber width and fiber length are all significantly reduced at 5 dpf in the embryos injected with cited3 S2-MO (P,1024). (C) The values of each measurement in the morphants (yellow) are normalized to that of the wild-type values (blue). The heights of the somites are also reduced. |
|
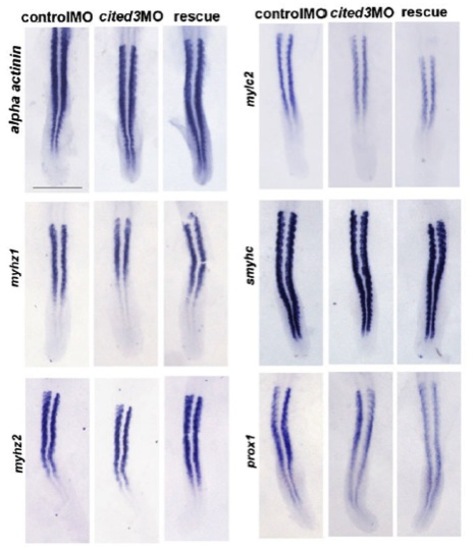
In situ hybridization results for representative genes that are expressed in the somites at 19 hpf. Expression levels of alpha actinin, myhz1, myhz2, mylc2, smyhc and prox1 were not affected in cited3 morphants at 19 hpf. |
|
Cited3 non-cell-autonomously regulates differentiation of fast muscle cells. (A–C) A fast myofiber transplanted from wild-type into a cited3 morphant could not express the fast myosin heavy chain at high levels. (D–F) A single fast myofiber transplanted from a cited3 morphant into wild-type host environment expresses the fast myosin heavy chain as high as its neighbor wildtype fast myofibers. Red is rhodamine dextran dye, green is fast myofiber specific myosin heavy chain expression that is detected by F310. Images are from trunk somites of the embryo. |