- Title
-
Nkx genes regulate heart tube extension and exert differential effects on ventricular and atrial cell number
- Authors
- Targoff, K.L., Schell, T., and Yelon, D.
- Source
- Full text @ Dev. Biol.
|
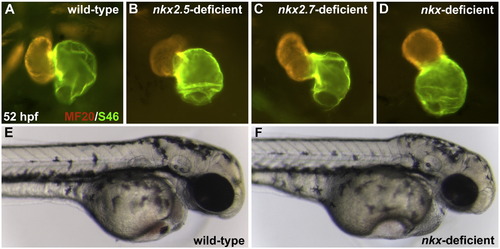
Knockdown of nkx2.5 and/or nkx2.7 causes prominent defects in cardiac chamber morphogenesis. (A–D) Frontal views, dorsal to the top at 52 hpf. MF20/S46 immunofluorescence distinguishes ventricular myocardium (red) from atrial myocardium (yellow). In comparison to wild-type embryos (A), injection of anti-nkx2.5 ATG MO (B) or anti-nkx2.7 ATG MO (C) causes subtle abnormalities in chamber size and shape. (D) Injection of anti-nkx2.5 and anti-nkx2.7 ATG MOs together reveals more striking defects in both ventricular and atrial morphology. (E,F) Lateral views of live embryos, anterior to the right, at 52 hpf. Other than their cardiac defects, pericardial edema, brain ventricle swelling, and jaw hypoplasia, nkx-deficient embryos appear morphologically normal. |
|
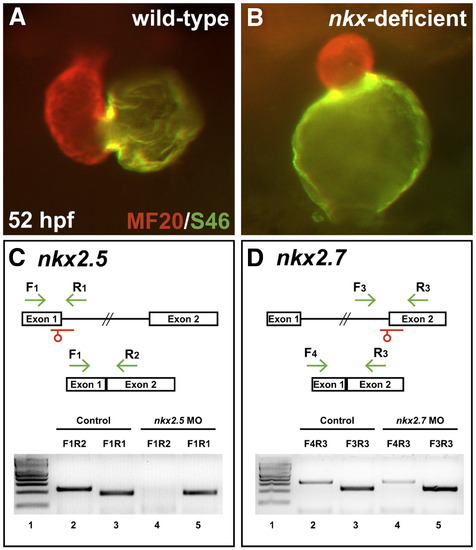
Splice MOs confirm specificity and efficacy of nkx2.5 and nkx2.7 knockdown. (A,B) Frontal views, dorsal to the top at 52 hpf. MF20/S46 immunofluorescence distinguishes ventricular myocardium (red) from atrial myocardium (yellow). Injection of anti-nkx2.5 and anti-nkx2.7 splice MOs has effects similar to that of injection of anti-nkx2.5 and anti-nkx2.7 ATG MOs (Fig. 1D). (C,D) MO injection impairs splicing of nkx2.5 and nkx2.7. Schematics depict the structures of the nkx2.5 (C) and nkx2.7 (D) pre-mRNA and mRNA. Locations of splice MOs are indicated in red. Primer pairs used for RT-PCR are indicated in green. Gels document RT-PCR amplification products, comparing detection of spliced (lanes 2 and 4) and unspliced (lanes 3 and 5) messages in cDNA from 26 hpf control embryos and embryos injected with anti-nkx2.5 splice MO or anti-nkx2.7 splice MO, respectively. Primer pair F1R1 amplifies 189 bp of unspliced nkx2.5 pre-mRNA, primer pair F1R2 amplifies 230 bp of spliced nkx2.5 mRNA, primer pair F3R3 amplifies 229 bp of unspliced nkx2.7 pre-mRNA, and primer pair F4R3 amplifies 323 bp of spliced nkx2.7 mRNA. In both gels, lane 1 contains a 100 bp molecular weight ladder (NEB, Ipswich, MA, USA). Embryos injected with anti-nkx2.5 splice MO exhibit a loss of spliced nkx2.5 mRNA, and embryos injected with anti-nkx2.7 splice MO demonstrate a partial reduction in spliced nkx2.7 mRNA with a concomitant increase in unspliced pre-mRNA. Note that the predicted PCR products with intronic inclusion for the unspliced nkx2.5 (1.4 kb) and nkx2.7 (2.2 kb) pre-mRNAs are absent, most likely because PCR conditions did not favor amplification of larger products. |
|
Bilateral populations of cardiomyocytes are normal in nkx-deficient embryos. In situ hybridization depicts expression of cmlc2 (A,B) and vmhc (C,D) in wild-type (A,C) and nkx-deficient (B,D) embryos. Embryos viewed dorsally, anterior to the top, at the 18-somite stage. Aside from a mild delay in cardiomyocyte migration toward the midline, nkx-deficient embryos appear to have the same patterns of cmlc2 and vmhc expression as are observed in wild-type embryos. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Cardiac cone formation occurs normally in nkx-deficient embryos. In situ hybridization depicts expression of cmlc2 (A,B), vmhc (C,D), and amhc (E,F) in wild-type and nkx-deficient embryos. Embryos viewed dorsally, anterior to the top, at the 22-somite stage. (A,C,E) Wild-type embryos exhibit normal fusion of the bilateral cardiac precursors, creating an intact cardiac cone. (B,D,F) nkx-deficient embryos mirror the expression patterns observed in wild-type, with the exception of a slightly enlarged and elongated lumen within the cardiac cone. EXPRESSION / LABELING:
PHENOTYPE:
|
|
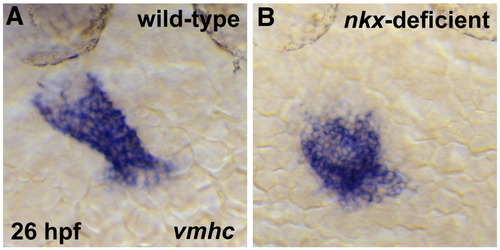
nkx genes play a critical role in early steps of heart tube extension. In situ hybridization depicts expression of cmlc2 (A,B), vmhc (C,D), and amhc (E,F) in wild-type (A,C,E) and nkx-deficient (B,D,F) embryos. Embryos viewed dorsally, anterior to the top, at 26 hpf. (A,B) nkx-deficient embryos exhibit prominent defects in heart tube extension, including a splayed and disorganized inflow region and an unusually compact outflow region. (C,D) In nkx-deficient embryos, the ventricular portion of the heart tube is abnormally short and wide. (E,F) In nkx-deficient embryos, the atrial portion of the heart tube is broad and sprawling. |
|
nkx genes limit atrial cell number during heart tube assembly. (A,B) Immunofluorescence indicates nuclear localization of Mef2 (green) throughout the heart tube, facilitating counting of differentiated cardiomyocytes at 26 hpf. Atrial cells are indicated by the anti-Amhc antibody, S46 (red). Hearts are flattened with a cover slip to improve visualization of cardiomyocyte nuclei. (C) Quantification of cardiomyocyte nuclei in wild-type (n = 25) and nkx-deficient (n = 11) embryos reveals a statistically significant increase in atrial cell number in nkx-deficient embryos (p < 0.001, Student's t-test) and no significant difference in ventricular cell number. Bar graph indicates mean and standard error of each data set; asterisk indicates statistically significant difference from wild-type. |
|
nkx genes are necessary to establish sufficient ventricular cell number during cardiac chamber formation. (A,B) Immunofluorescence indicates that both chambers express the transgene Tg(cmlc2:DsRed2-nuc) (red), facilitating cardiomyocyte counting at 52 hpf. Atria are labeled with the anti-Amhc antibody, S46 (green). Hearts are flattened with a cover slip to improve visualization of cardiomyocyte nuclei. (C) Quantification of cardiomyocyte nuclei in wild-type (n = 10) and nkx-deficient (n = 13) embryos reveals a statistically significant increase in atrial cell number in nkx-deficient embryos (p < 0.001, Student's t-test) and a statistically significant decrease in ventricular cell number in nkx-deficient embryos (p < 0.001, Student's t-test). Bar graph indicates mean and standard error of each data set; asterisks indicate statistically significant differences from wild-type. |
|
Positive and negative controls for the RT-PCR experiment shown in Figs. 2C,D. (A) Positive controls using primers for β-actin demonstrate similar efficacy of cDNA synthesis in all samples. (B) Negative controls omitting reverse transcriptase from the cDNA synthesis reaction demonstrate absence of contaminating genomic DNA. |
|
Splice MOs recapitulate the phenotype resulting from injection of ATG MOs. In situ hybridization depicts expression of vmhc in (A) wild-type and (B) anti-nkx2.5 and anti-nkx2.7 splice MO-injected embryos. Embryos viewed dorsally, anterior to the top, at the 26 hpf stage. The vmhc-expressing portion of the nkx-deficient heart tube is substantially shorter and wider than the vmhc-expressing portion of the wild-type heart tube. |

Unillustrated author statements PHENOTYPE:
|
Reprinted from Developmental Biology, 322(2), Targoff, K.L., Schell, T., and Yelon, D., Nkx genes regulate heart tube extension and exert differential effects on ventricular and atrial cell number, 314-321, Copyright (2008) with permission from Elsevier. Full text @ Dev. Biol.