- Title
-
Inhibition of neural crest migration underlies craniofacial dysmorphology and Hirschsprung's disease in Bardet-Biedl syndrome
- Authors
- Tobin, J.L., Di Franco, M., Eichers, E., May-Simera, H., Garcia, M., Yan, J., Quinlan, R., Justice, M.J., Hennekam, R.C., Briscoe, J., Tada, M., Mayor, R., Burns, A.J., Lupski, J.R., Hammond, P., and Beales, P.L.
- Source
- Full text @ Proc. Natl. Acad. Sci. USA
|
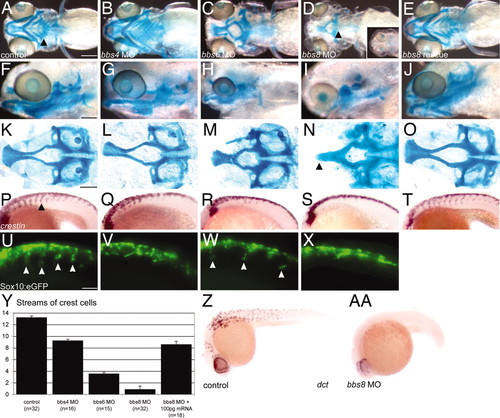
Craniofacial and neural crest defects in bbs zebrafish morphants. (A–E) Ventral views of wholemount, Alcian Blue-stained, 5-dpf zebrafish. bbs8 morphants are most severe (D), with loss of pharyngeal apparatus (2D arrowhead), mandibles, and shortening of the chondrocranium. (F–J) Lateral views of larvae showing reduction of branchial arches and shortening of the mandibles in bbs6 and bbs8 morphants. (K–O) Flatmounts of neurocrania of controls and morphants. bbs8 morphants often have fusion of the trabeculae at the midline (arrowhead) similar to syu mutants. (P–T) Crestin in situ in 20ss embryos reveals streams of migrating NCCs in controls (arrowhead) with progressively fewer streams in bbs4, bbs6, and bbs8 morphants, respectively. (U–X) Sox10:eGFP expression in migrating streams (arrowheads) shows a similar reduction in the number of streams to the crestin expression. (Y) Quantification of the number of streams in morphants confirms a severe neural crest migration defect in bbs8 morphants that can be rescued by human mRNA. (Z and AA) dct expression in 27 hpf control embryos (Z) and bbs8 morphants (AA). [Scale bars: 500 μm (A–J), 100 μm (K–O), and 100 μm (P–X).] EXPRESSION / LABELING:
PHENOTYPE:
|
|
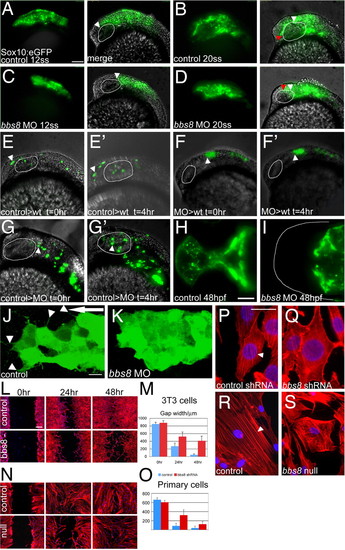
Neural crest migration in bbs8 morphants. (A and B) Sox10:GFP transgenic zebrafish showing migration of CNCCs anteriorly between 12 (A) and 20ss (B). The amount of movement over the 4-h period is represented in B by the difference between the white (position at 12ss) and red (position at 20ss) arrowheads, H250 μm. (C and D) Inhibition of CNCC migration in bbs8 morphants. Only a small distance (80 μm) moved in the 4-h period. (E and E2) NCCs from control MO-injected embryos grafted into control hosts migrate anteriorly to populate the head. The arrowhead shows the position of the anteriormost cell showing its change in position over the 4-h period. (F and F2) Cells taken from bbs8 morphant donors fail to migrate when transplanted into control hosts, suggesting that the migration defect is cell autonomous. (G and G2) Control cells transplanted into bbs8 morphant hosts migrate as normal. (H and I) Control and morphant embryos at 48 hpf showing Sox10:eGFP expression in the developing trabeculae that fail to elongate in morphants. (J) Migrating control CNCCs at 14ss showing polarized filopodial protrusions (arrowheads) mainly in the direction of migration (large arrow). (K) bbs8 morphant CNCCs remain tightly clustered and lack polarized protrusions. (L) In vitro scratch wound healing assay on 3T3 cells shows delayed closure of the gap in Bbs8 knockdown cells compared with controls. (M) Graph quantifying the gap width over the 48-h time course. (N) The same wound healing assay using primary fibroblasts taken from unaffected and BBS8 -/- patients also shows delayed migration. (O) BBS8 patient cells migrate slower than control. (P) Polarized actin microfilaments in control 3T3 cells migrating into the wound gap. (Q) Bbs8 knockdown cells have a disordered actin cytoskeleton. (R and S) This disorder is recapitulated in migrating patient primary fibroblasts. [Scale bars: 100 μm (A–G2), 100 μm (H and I), 20 μm (J and K), 200 μm (L and N), and 20 μm (P–S).] EXPRESSION / LABELING:
PHENOTYPE:
|
|
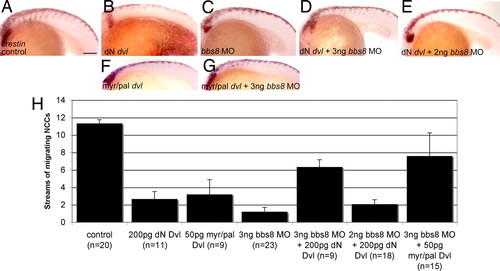
Noncanonical Wnt signaling and NC migration. (A–C) Injection of both dN dvl mRNA and bbs8 MO individually results in inhibition of NCC migration relative to controls. (D) Coinjection of 3 ng of bbs8 MO with dN dvl results in partial rescue of NCC migration by H4-fold relative to bbs8 morphants. (E) Coinjection of dN dvl mRNA with 2 ng of bbs8 MO fails to rescue NCC migration. (F) Injection of 50 pg of myr/pal dvl causes inhibition of NCC migration. (G) Coinjection of myr/pal dvl with 3 ng of bbs8 MO also partially rescues the inhibited NC migration. (H) Graph quantifying the number of streams of crestin-positive NCCs migrating through the trunk. (Scale bar: 200 μm.) EXPRESSION / LABELING:
|
|
Enteric nervous system development in bbs morphants. (A) Control embryos at 4 dpf showing enteric neurons populating the length of the gut from the anterior (left-hand side) to the anus (arrowhead). (B) With 2 ng of bbs8, MO migration of enteric neurons ceases midway along the gut (asterisk), and no neurons reach the anus (arrowhead). (C) Four nanograms of bbs8 MO causes complete absence of enteric neurons, resulting in gut motility defects (see Movie S4 and Movie S5). (D) Quantification of the reduction in number of enteric neurons with progressively higher doses of bbs8 MO. (E and F) NCCs exiting the vagal region visualized by crestin show no difference between control and morphant. (G and H) phox2b expression at 48 hpf shows a reduced number of NCCs in the branchial arches. (I and J) Lateral view of the embryos in G and H shows a failure of cells to leave the pharyngeal arches and enter the gut tube. (K) phox2b-positive enteric neurons populating the entire length of the gut all the way to the anus. The arrowhead shows the posteriormost extent of migration, and the asterisk represents the anus. (L and M) Injection of 2 ng of, and 4 ng of, bbs8 MO, respectively, causes neurons to migrate only halfway along the gut (2 ng) or fail to enter the gut at all (4 ng). [Scale bars: 500 μm (A–D), 100 μm (E and F), 200 μm (G–J), and 300 μm (K–M).] EXPRESSION / LABELING:
|
|
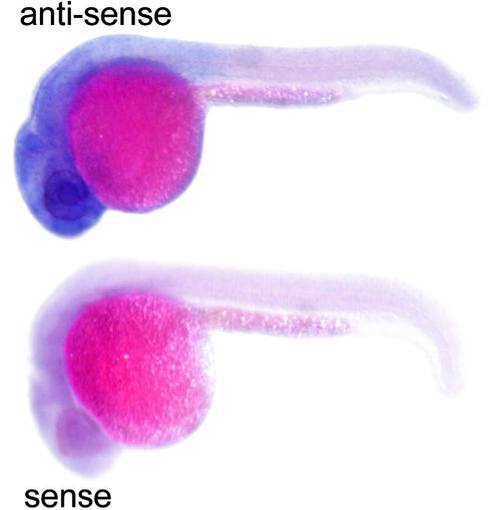
bbs8 expression pattern in zebrafish. In situ hybridization showing ubiquitous expression of bbs8 in the 24 hpf embryo with high levels in the eye and brain. EXPRESSION / LABELING:
|
|
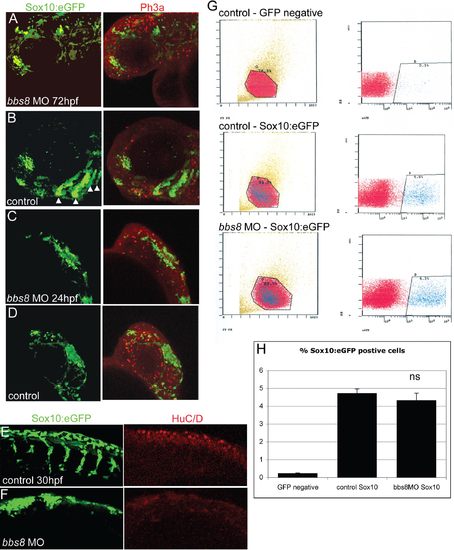
Assessment of proliferation, cell death, and differentiation in bbs8 morphant NC. (A and B) Expression of Sox10:eGFP in 72 hpf zebrafish shows a lack of pharyngeal arches in morphants relative to controls (arrowheads in B). Also, the number of proliferating Sox10:eGFP-positive cells shown by Ph3 staining (in red) is not different between control and morphant. (C and D) Sox10:eGFP expression in the head of 24 hpf fish showing the lack of migration in bbs8 morphants. Ph3 staining again shows no difference in the amount of proliferation in the crest cells despite less proliferation in cells of the brain. (E and F) Sox10:eGFP cells migrating into the trunk at 30 hpf do not inappropriately differentiate into neurons in the bbs8 morphants as revealed by HuC/D immunostaining (in red). (G and H) FACS plots (G) of embryos show that control and morphant Sox10:eGFP embryos have similar proportions of neural crest cells (H), indicating that proliferation of the NCCs is not affected in the morphants. EXPRESSION / LABELING:
|
|
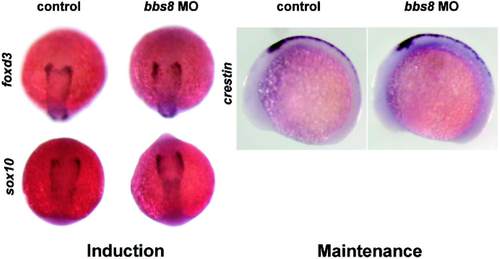
Expression pattern of neural crest markers. Expression of sox10 and foxd3 were normal, proving that NCC induction was normal at 5 somite stage. Normal expression of crestin at 12 somite stage indicates that NCC maintenance and proliferation are also normal in morphants. EXPRESSION / LABELING:
|
|
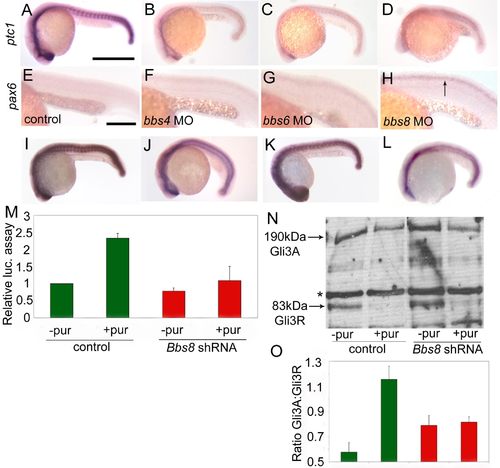
Aberrant Sonic Hedgehog signaling in bbs morphants. (A) Expression of ptc1, a marker of Sonic Hedgehog pathway activity, is down-regulated in bbs morphants, most strikingly so in bbs8 morphants (6D). (E and F) Conversely, pax6, a gene negatively regulated by Shh signaling, is up-regulated in the neural tube of bbs8 morphants. (I) Injection of ptc1 MO alone up-regulates expression of ptc1 by inducing the Shh pathway. (J) Coinjection of ptc1 MO with bbs8 MO partially rescues this induction, implicating Bbs8 downstream of Ptc1. (K) Injection of dominant-negative PKA causes ectopic activation of the Shh pathway. (L) Coinjection with bbs8 MO rescues this induction, suggesting that it could act downstream of PKA, likely in Gli3 transport. (M) Addition of Purmorphamine to control transfected cells induces the Gli activity by 2.5-fold. Bbs8 knockdown cells show attenuated response to Purmorphamine stimulation. (N and O) Western blot showing reduction of Gli3R in response to stimulation with Purmorphamine, resulting in a higher Gli3A;Gli3R ratio. This ratio remains unchanged when Bbs8 knockdown cells are stimulated. [Scale bars: 500 μm (A–D and I–L) and 200 μm (E–H).] EXPRESSION / LABELING:
|
|
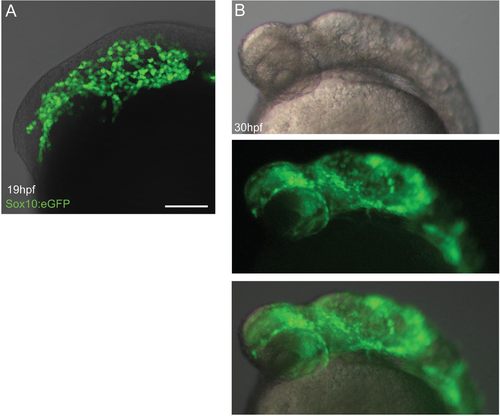
Effect of cyclopamine treatment on early NCC migration. (A) Adding cyclopamine at 4 hpf causes no defect in CNCC migration at 19 hpf shown by Sox10:eGFP expression. (B) At 30 hpf, the embryos show morphological signs of Shh pathway inhibition, but CNCCs have still migrated into the head, showing that Shh signaling is not important for initial migration of cells but is critical for patterning. EXPRESSION / LABELING:
|