- Title
-
Olfactomedin-2 mediates development of the anterior central nervous system and head structures in zebrafish
- Authors
- Lee, J.A., Anholt, R.R., and Cole, G.J.
- Source
- Full text @ Mech. Dev.
|
Identification and expression of zebrafish olfactomedin 2. (A) Schematic representation of the zebrafish OM2 gene. The first exon includes the sequence obtained by 5′ RACE. The gray box at the 5′ end indicates a signal sequence-coding sequence predicted by the SignalP3.0 algorithm (http://www.cbs.dtu.dk/services/SignalP/). Numbers in exons (boxes) indicate the numbers of nucleotides. The positions targeted by the two MOs used in this study are indicated by black lines. Arrows indicate the primers used for RT-PCR to verify the efficiency of 3i4e MO (D). Intron lengths are not to scale. (B) Coding sequence of OM2 first exon and corresponding amino acid sequence. The underlines on the amino acid sequence indicate signal peptides predicted by SignalP 3.0 server using neural networks (top line) and hidden Markov model (bottom line). (C) Analysis of OM2 mRNA expression during zebrafish development using RT-PCR. (D) RT-PCR with cDNA from 37 hpf fish shows that the intron–exon boundary targeting morpholino (3i4e MO) specifically reduces mRNA with the correct splicing at the 3rd intron and 4th exon junction. OM2 primers used for this experiment are indicated in (A) (arrows). As a control, zebrafish actin mRNA level was not altered by OM2 3i4e MO, which indicates that disruption of mRNA splicing was specific for the target sequence. |
|
Localization of OM2 mRNA in developing zebrafish using in situ hybridization. (A) OM2 mRNA is not detected in 10 hpf embryos. (B and C) Embryo (19 hpf) shows OM2 mRNA in the developing eye and branchiomotor nuclei (III, V, VII and X). Expression in the proximal region of the optic primordium is shown in the inset (yellow dotted line shows the boundary of optic primordium). (D and E) In 22.5 hpf embryos broader domains of OM2 mRNA expression were detected as well as the sharply defined branchiomotor nuclei-specific expression. GFP expression in branchiomotor nuclei of an islet-GFP embryo is also shown in (E) demonstrating the branchiomotor identity of these nuclei. Arrowhead in (D) indicates a strong expression of OM2 in the tectum, which is also observed in a cryostat section (arrowhead in the inset). (F and G) OM2 expression pattern in a 27 hpf embryo. Strong expression is detected in the forebrain and the tectum as shown in G. (H and I) Embryo (36 hpf) shows strong expression of OM2 mRNA in the anterior CNS, including ventral regions that will develop into the lower jaw and pharyngeal arches (arrows in H). In addition, OM2 is clearly detected in the eyes. (J and K) Embryos (48 hpf), showing OM2 expression in eye and brain, the epiphysis (arrow in J), with branchiomotor nuclei expression still being observed (J). (L and M) In 54 hpf embryos OM2 expression in forebrain, tectum, epiphysis and eyes is detected. More stratified patterns of OM2 mRNA are observed in the inner nuclear cell layer (in) and retinal ganglion cell layer (rg) in the eye (M). Close observation with cryosections shows that OM2 mRNA is still expressed in vagal (CN X) branchiomotor nuclei (arrows in N: red dotted box in M indicates the region shown in cryosection N). OM2 in developing pharyngeal arches is more restricted and this pattern is continued to 70 hpf embryos. (O and P) Embryo (70 hpf) shows a similar pattern to 54 hpf embryos. Notice a strong, yet highly restricted pattern of OM2 mRNA expression is found along the pharyngeal arches (red inset in O was obtained from an oblique angle). Also in cryosections of 70 hpf embryonic eye OM2 mRNA is expressed in defined, laminated layers of the INL and RGC, as in 54 hpf embryos (inset in P). Small arrowheads in P indicates OM2 mRNA expression in pharyngeal arches. EXPRESSION / LABELING:
|
|
OM2 morphants show specific defects in anterior CNS development (n = 78). (A and B) Embryos (25 hpf) from the lateral view show specific defects of OM2 morphants in the midbrain–hindbrain boundary (MHB), which appears to have been formed by the fusion of tectal (arrow) and cerebellar walls (arrowheads). Slightly smaller size of the morphant eyes is also shown. (C and D) At 37 hpf, the MHB defect is still present. (E–H) Pax2a in situ hybridization patterns are not affected by OM2 knockdown. Asterisks indicate otic vesicles. Pax2a mRNA expression at the MHB is intact in OM2 morphants (arrowheads), despite the morphological defects (B and D). (I and J) MHB-specific Fgf8 mRNA expression pattern is not altered by OM2 knockdown. Arrowheads indicate Fgf8 mRNA at the MHB. (K and L) At 60 hpf, gross morphology of OM2 morphants is similar to that of control MO injected fish, except for mild edema in the 4th ventricle (arrow in L). (M and N) Dorsal side of the head shows markedly decreased size of the optic tectum (asterisks). Notice also that the MHB is significantly thinner and less developed than its control counterpart. (O and P) In situ hybridization with SHH probes shows no alteration in SHH mRNA expression patterns in OM2 morphants (arrows; n = 24). EXPRESSION / LABELING:
|
|
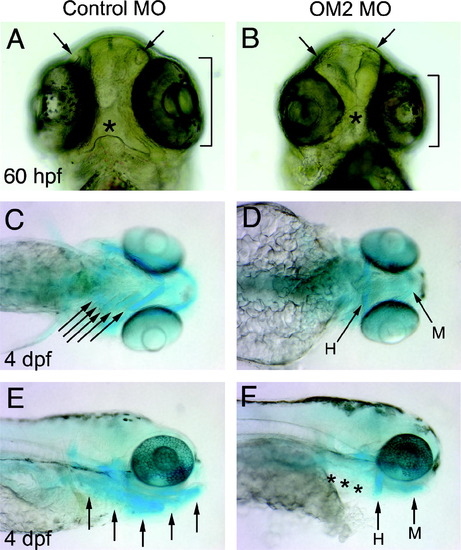
Craniofacial abnormalities in OM2 morphants. (A and B) Ventral view of the head shows several defects (n = 170). Arrows point at underdeveloped olfactory pits. Brackets indicate smaller eyes (A and B). Asterisk shows a malformed mouth. C–F Alcian blue staining of 4 dpf morphants shows the severely impaired pharyngeal arch cartilages (n = 27). Arrows in (C) indicate cartilages in developing pharyngeal arches, which appear absent in OM2 morphants. H, hyoid arch; M, mandibular arch. In (E) and (F) the dramatically reduced cartilage in the pharyngeal arch is again apparent (asterisks in F). |
|
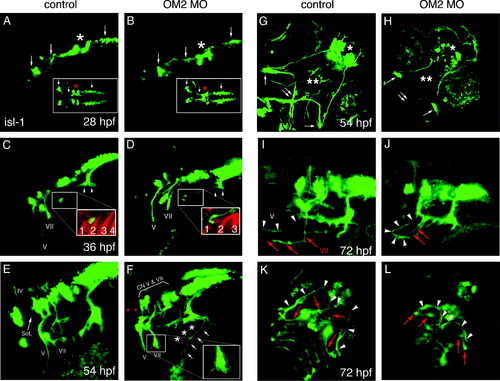
Axon guidance of branchiomotor neurons is perturbed in OM2 morphants. Head is to the left in A–F. All images were captured by a laser confocal microscope and z-stacked images were collapsed. (A and B) Lateral and dorsal view of 28 hpf embryos showing branchiomotor nuclei and initial axon outgrowth (n = 23 for A, n = 40 for B). Arrows show newly formed branchiomotor axon bundles and primary branchiomotor neurons. No apparent defects were detected at 28 hpf, except for fusion of the trigeminal nuclei in OM2 morphants (asterisks). (C and D) Embryos (36 hpf), showing normal development of branchiomotor nuclei in morphants (D), but mildly impaired outgrowth of branchiomotor axons (n = 20 for C, n = 44 for D). Inset in D shows failure of axons to grow into the pharyngeal arches in OM2 morphants (red: zn5 staining). Arrowheads in (C) indicate newly formed gill motor axons. Numbers in insets of (C and D) show pharyngeal endodermal pouches, absence/fusion of pouches in morphants. (E–H) Embryos (54 hpf) analyzed for branchiomotor development (n = 25 for E and G, n = 73 for F and H). In control islet-1-GFP fish (E), cranial nerve IV, V and VII are labeled with Roman numerals. SoL indicates superior orbital lateral line axons. (F) Lateral view of branchiomotor axons of a 54 hpf ATG OM2 morphant fish. Islet-1-derived GFP expression shows the stereotypically perturbed projection of cranial nerve V and VII with abrupt endings and numerous fine filopodia (inset in F). Gill motor neurons from CN X do not form nerve bundles that innervate gill muscles (asterisks in F). Despite the relatively normal position of branchiomotor nuclei V and VII, close observation reveals slightly hypomorphic nuclei in morphants (bracket with CN V and VII in F). Aberrant axons with anteriorily wandering processes were detected from the vagus (X) nerve, as indicated by arrows in (F). Absence of SoL is indicated by red asterisks. (G) Ventral view of 54 hpf control fish. The characteristic crossed axon pathway formed by cranial nerve V and VII is apparent (double arrow). Single arrows indicate intermediate branches of cranial nerve V. (H) In OM2 ATG morphants cranial nerve V and VII prematurely terminate as shown in (F), which results in the absence of the crossed axon pathway (double arrow). Notice also the absence of peripherally extended cranial nerve VII in morphants (double asterisk). Single asterisk indicates hypomorphic telencephalic nuclei. (I) Lateral view of 72 hpf control fish. Anteriorily projected CN V and VII are indicated by white arrowheads (V) and red arrows (VII). (J) Lateral view of 72 hpf OM2 morphant fish. One of the well-recovered fish is shown here. CN V axon (white arrowheads) grew further compared with (F) and made a stereotypic medio-caudal turn, whereas CN VII axons did not grow much further (red arrows). (K) Ventral view of 72 hpf control fish. As in (G) a cross-shaped axon pathway made by CN V and CN VII axons is readily visible (white arrowheads and red arrows). (L) Ventral view of 72 hpf OM2 morphant. White arrowheads indicate the axon pathway formed by further-projected CN V axons, which could reach the midline in this case. Notice much more caudal location of the axon pathway compared with that in control fish (K). Red arrows indicate CN VII axons that did not grow further from the earlier observation (54 hpf ventral view, H). |
|
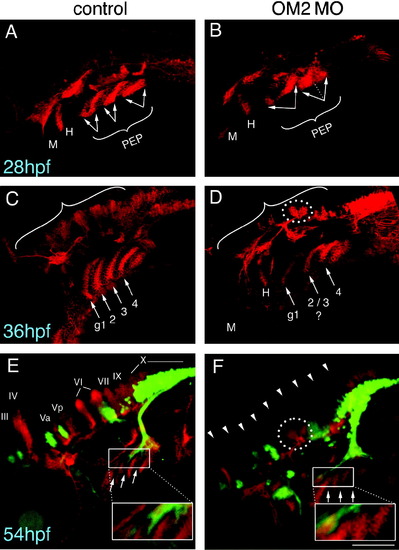
Formation of dorso-laterally located commissural nuclei is disrupted in OM2 morphants (number of samples analyzed are the same as in Fig. 5). Later-onset, ascending commissural nuclei within the hindbrain were visualized using Zn-5 immunostaining. Islet-1 positive primary motor neurons are detected by GFP (green). (A, C and E) Control embryos. (B, D and F) OM2 morphants. (A and B) Embryos (28 hpf), showing that pharyngeal arch endodermal pouch (PEP) formation is moderately defective in OM2 morphants (arrows). Dotted line in (B) denotes partially divided endodermal pouches in OM2 morphants. M, mandibular arch; H, hyoid arch. (C and D) Embryos (36 hpf), showing intricate structures of Zn-5 positive cells. Hindbrain commissural neurons (brackets) are almost completely lacking or severely underdeveloped in OM2 morphants, although commissural neurons adjacent to the CN VI do form partially in morphants (dotted circle). PEP formation in OM2 morphants also appears abnormal, with pharyngeal endodermal pouches 2 and 3 possibly fused. g1, first gill endodermal pouch. (E and F) In 54 hpf embryos continued absence of development of hindbrain commissural neurons is observed in OM2 morphants. Gill motor axons from cranial nerve X send their axons through Zn-5 negative areas (inset in E), which is sharply contrasted by morphant embryos where gill motor axons fail to grow through the PEPs (inset in F). All photographs were taken with 200x magnification and z-stacked images were flattened to show structures located at different depths. The scale bar in (F) indicates 100 micrometer. |
|
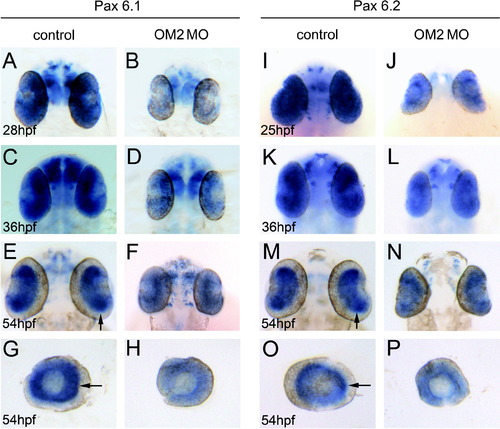
Pax 6 mRNA expression is disrupted in OM2 morphants. (A, C, E and G) Pax6.1 in situ hybridization of 28–54 hpf control fish eyes. (B, D, F and H) Pax6.1 in situ hybridization of 3i4e MO-injected fish eyes. Notice the reduction of Pax6.1 mRNA level in the eye at all ages, but Pax6.1 mRNA can still be observed in the brain. Arrows in (E) and (G) indicates a sharply delineated Pax6.1 mRNA expression pattern at 54 hpf, which was lost in OM2 morphant eyes (F and H). (I, K, M and O) Pax6.2 in situ hybridization of 25–54 hpf control fish. High level of Pax6.2 mRNA expression is particularly evident in the 54 hpf eye with sharp, circular boundaries (arrows in M and O). (J, L, N and P) Pax6.2 in situ hybridization of 25–54 hpf 3i4e MO-injected fish. Much weaker Pax6.2 mRNA expression with less well-defined layers is observed in the eye at all developmental stages. |
|
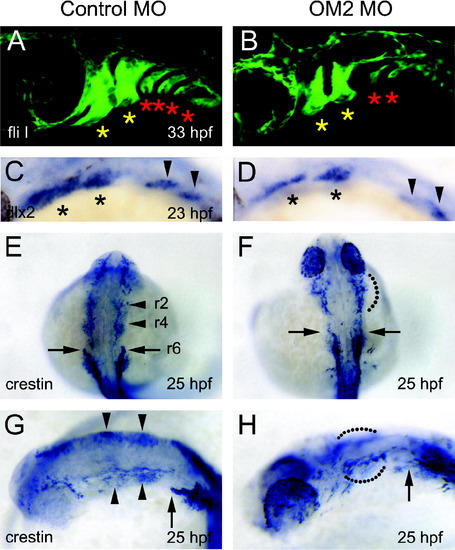
Analysis of cranial neural crest cells in OM2 morphants. (A and B) Confocal micrographs of 33 hpf fliI-GFP fish. Head is left. The first and second (yellow) asterisks indicate the developing mandibular and hyoid arches, which are only mildly affected by OM2 knockdown. More caudal pharyngeal arches (red asterisks) are severely affected in OM2 morphants. (C and D) dlx2 in situ hybridization of 23 hpf fish. Asterisks in (C) and (D) indicate the migrated cranial neural crest cells within the developing mandibular and hyoid arches. Cranial neural crest cells in more caudal pharyngeal arches are indicated by arrowheads. Although hypomorphic, the pattern and position of the dlx2-positive cells suggest that initial specification and migration of cranial neural crest cells are normal in OM2 morphants. (E–H) Crestin in situ hybridization of 25 hpf zebrafish. Rostral streams of cranial neural crest cell migration are indicated by r2, r4 and r6, which originate from rhombomeres 2, 4 and 6, respectively. In morphants (F and H), r2 and r4 streams were not well segregated (68/70: dotted lines) and r6 stream was hypomorphic (69/70). |
Reprinted from Mechanisms of Development, 125(1-2), Lee, J.A., Anholt, R.R., and Cole, G.J., Olfactomedin-2 mediates development of the anterior central nervous system and head structures in zebrafish, 167-181, Copyright (2008) with permission from Elsevier. Full text @ Mech. Dev.