- Title
-
The atypical Rho GTPase, RhoU, regulates cell-adhesion molecules during cardiac morphogenesis
- Authors
- Dickover, M., Hegarty, J.M., Ly, K., Lopez, D., Yang, H., Zhang, R., Tedeschi, N., Hsiai, T.K., Chi, N.C.
- Source
- Full text @ Dev. Biol.
|
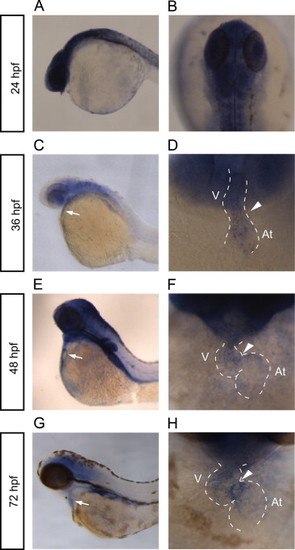
rhoua is expressed in the heart and brain. (A–H) Whole mount RNA in situ hybridization reveals rhoua expression in the zebrafish at (A, B) 24 hpf, (C, D) 36 hpf, (E, F) 48 hpf, and (G, H) 72 hpf. (A, C, E, G) Lateral view of the zebrafish head and torso. White arrow points to heart. (B) Dorsal view of 24 hpf zebrafish head. (D, F, H) Ventral view of heart. Anterior is to the top. Dashed lines outline the heart. Arrowhead points to AV canal. At – atrium, V – ventricle. EXPRESSION / LABELING:
|
|
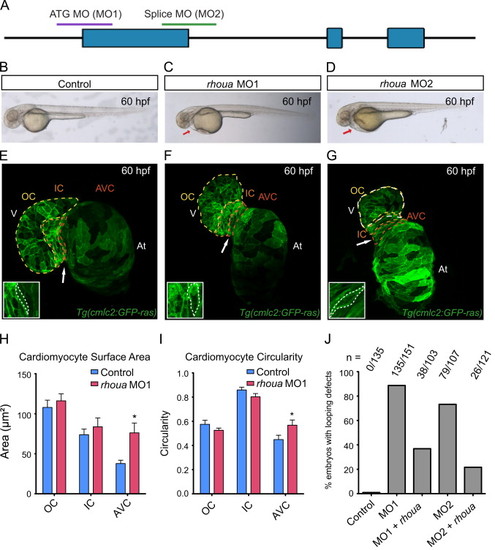
Loss of rhoua results in cardiac edema due to morphologic atrioventricular (AV) canal defects. (A) The following regions of rhoua were targeted to create the rhoua ATG (MO1) and splice (MO2) morpholinos (MO). (B–D) Brightfield microscopy imaging shows that rhoua ATG and splice MO injected embryos (C, D) exhibited cardiac edema (red arrows) by 60 hpf, but control MO injected embryos (B) did not. (E–G) Confocal projections of 60 hpf Tg(cmlc2:ras-eGFP) (E) control MO, (F) rhoua ATG MO1, and (G) rhoua splice MO2 injected zebrafish revealed that rhoua MO injected zebrafish hearts failed to undergo proper cardiac looping and displayed AV canal defects. White arrow points to AV canal. Outer curvature (OC), inner curvature (IC), and atrioventricular canal (AV) are outlined in yellow, orange, and red dashed lines, respectively. At– atrium, V – ventricle. Inset shows representative AV cardiomyocytes, which appear more rounded and larger in MO injected embryos compared to control embryos. (H, I) Bar graphs represent cardiomyocyte (H) surface area and (I) morphology/circularity measurements of control and ATG MO1 Tg(cmlc2:ras-eGFP) hearts at 60 hpf. Mean+s.e.m. Studentós t-test, *p<0.05 (n=5 control and 7 rhoua morpholino injected zebrafish hearts). (J) 88.7% and 73.3% of zebrafish embryos injected with a rhoua ATG (MO1) and splice (MO2) MOs exhibited cardiac edema and AV canal defects, but wild-type embryos injected with control MO did not (0%). Injection of 150 pg of wild-type zebrafish rhoua was able to rescue the cardiac phenotype in the rhoua ATG and splice MO injected zebrafish and reduce the mutant phenotype to 36.9% and 21.7% for ATG MO1 (MO1+rhoua) and splice MO2 (MO2+rhoua), respectively. n indicates the number of embryos with cardiac phenotype/total number of embryos injected for each condition. |
|
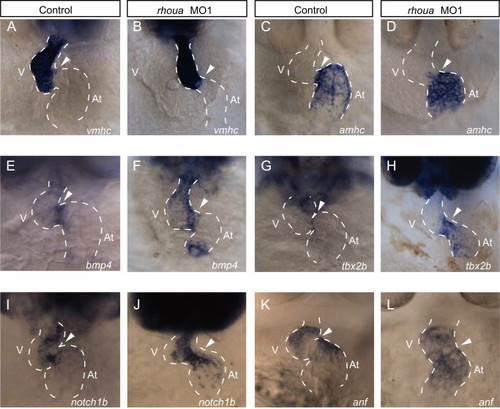
rhoua knockdown does not affect cardiac cell fate specification and patterning. In situ analysis of AV and chamber specific cardiac genes revealed that cardiac cell fate specification and patterning were not perturbed in rhoua ATG MO injected zebrafish embryos. (A, B) vmhc and (C, D) amhc expression were restricted to the ventricular and atrial chambers, respectively, in (A, C) control and (B, D) rhoua MO1 knockdown embryos. (E, F) bmp4, (G, H) tbx2b, and (I, J) notch1b AV canal markers appeared to be expressed more broadly in the AV canal of (F, H, J) rhoua MO1 injected embryos when compared to (E, G, I) control MO injected embryos. (K, L) Conversely, anf was expressed in the chambers but not at the AV canal in (K) control and (L) rhoua MO injected embryos. Ventral view of heart. Anterior is to the top. Dashed lines outline the heart. Arrowhead points to AV canal. At – atrium, V – ventricle. EXPRESSION / LABELING:
|
|
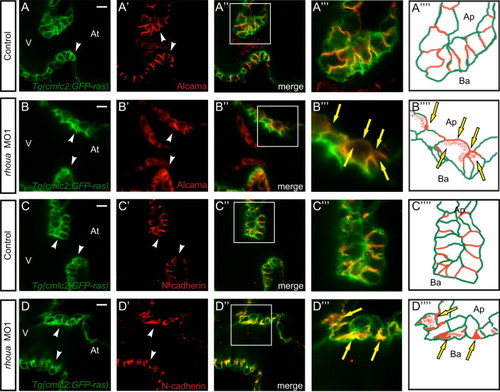
The cell adhesion proteins in the AV cardiomyocytes of rhoua morpholino knockdown zebrafish hearts are mis-localized. Confocal imaging of Tg(cmlc2:ras-eGFP) control and rhoua ATG MO1 knockdown zebrafish hearts that were examined by (A, B) Alcama antibody and (C, D) N-Cadherin antibody at 60 hpf reveals mislocalization of Alcama and N-cadherin in rhoua MO1 knockdown zebrafish AV cardiomyocytes. (A-D) Tg(cmlc2:ras-eGFP)s883 AV cardiomyocytes, (A′-D′) Immunohistochemical staining of AV cardiomyocytes, (A′′-D′′) Merge of Tg(cmlc2:ras-eGFP)s883 and immunohistochemical staining analysis, (A′′′-D′′′) Boxed area of A′′-D′′, and (A′′′′-D′′′′) Schematic representation of A′′′-D′′′. White arrowhead - AV cardiomyocytes, yellow arrows - mislocalized Alcama and N-cadherin immunostaining. At - atrium, V - ventricle, Ap - apical, Ba - basal. White scale bar - 10 µM. EXPRESSION / LABELING:
|
|
Arhgef7b is required to localize N-cadherin and Alcama to the cell junctions. (A, E) Brightfield microscopy showed that (E) the arhgef7b mutant, bubblehead (bbh), exhibited cardiac edema (red arrow) and cranial hemorrhaging (asterisk) by 60 hpf. (B, F) Confocal projections of 60 hpf control wild-type sibling and bbh mutant phalloidin stained (F-actin) hearts revealed that bbh mutant hearts failed to undergo proper cardiac looping and displayed AV canal defects. White arrowhead points to AV canal. (C, D, G, H) Immunohistochemical staining of control and bbh mutant hearts at 60 hpf using (C, G) Alcama and (D, H) N-Cadherin antibodies, revealed mislocalization of Alcama and N-cadherin in bbh mutant AV cardiomyocytes (G, H – white arrow). At – atrium, V – ventricle. (I) bbh/arhgef7b genetically interacts with rhoua. Cardiac edema and AV canal defects were observed more frequently in bbh +/ embryos than in wild-type embryos upon increasing titrated doses of rhoua MO1. (WT+2.5, 5, 7.5, 10 ng MO1 – 0%, 21.4%, 52.7%, 88.7% cardiac phenotype and arhgef7b+/m292+2.5, 5, 7.5, 10 ng MO1 – 15.4%, 37.5%, 78.9%, 100% cardiac phenotype). |
|
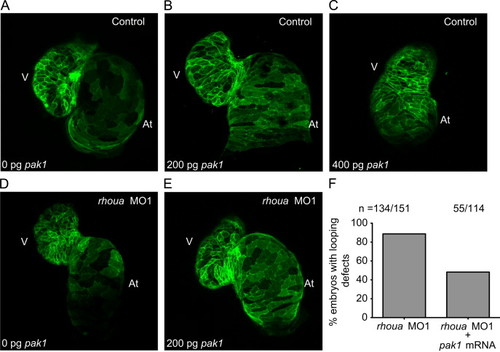
Pak1 is able to rescue the rhoua MO injected zebrafish cardiac phenotype, but high dose Pak1 results in dysmorphic hearts. (A–C) Although injection of 200 pg of pak1 RNA into control MO Tg(cmlc2:ras-eGFP)s883 zebrafish embryos did not cause any significant cardiac phenotypes, injection of 400 pg of pak1 resulted in dysmorphic hearts. Tg(cmlc2:ras-eGFP)s883 (A) 0 pg pak1 RNA, (B) 200 pg pak1 RNA, and (C) 400 pg pak1 RNA injected into control MO embryos. (D, E) Injection of 200 pg of pak1 RNA was able to rescue the cardiac phenotype in the rhoua MO1 knockdown zebrafish. Tg(cmlc2:ras-eGFP)s883 (D) rhoua MO1 and (E) rhoua MO1+200 pg pak1 RNA injected embryos. (F) 88.7% of rhoua MO1 knockdown zebrafish embryos exhibited cardiac edema and AV canal defects, but injection of 200 pg of pak1 RNA reduced the cardiac phenotype to 48.2%. n indicates the number of embryos with cardiac phenotype/total number of embryos injected for each condition. At – atrium, V – ventricle. |
|
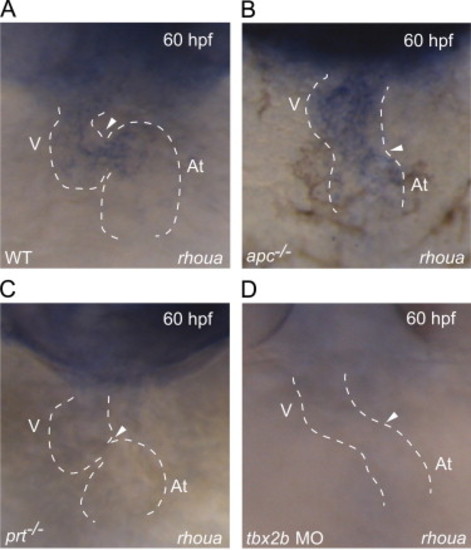
rhoua is misexpressed in apc, prt and tbx2b mutants. (A–D) Whole mount RNA in situ hybridization shows that rhoua is expressed at the AV canal at 60 hpf in (A) wild-type hearts, throughout (B) apc mutant hearts, but absent at the AV canal in (C) prt mutant and (D) tbx2b morphant hearts. Ventral view of heart. Anterior is to the top. Dashed lines outline the heart. Arrowhead points to AV canal. At – atrium, V – ventricle. EXPRESSION / LABELING:
|
|
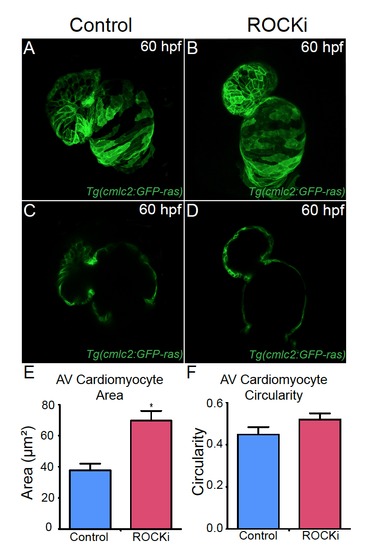
ROCK inhibitor treated hearts exhibit AV canal defects. (A, B) Confocal projections and (C, D) cross-sectional analyses of 60 hpf Tg(cmlc2:ras-eGFP) hearts reveal that (B, D) ROCK inhibitor (ROCKi) treated zebrafish exhibit cardiac looping and AV canal defects. (E, F) Bar graphs represent cardiomyocyte (E) surface area and (F) morphology/circularity measurements of Tg(cmlc2:ras-eGFP) hearts at 60 hpf. Mean+s.e.m. Studentós t-test, *p<0.05 (n = 12 for control DMSO-treated hearts and n = 12 for ROCK inhibitor treated hearts). |
|
rhouaATG morpholino (MO) represses GFP expression of arhoua-GFPreporter construct andrhouasplice MO perturbs splicing of therhouagene. (A-D) rhoua ATG MO1 blocks GFP expression from a construct in which the target sequence is placed upstream of the GFP coding sequence (rhoua-ATG-GFP). (A, B) RNA injected from the rhoua-ATG-GFP construct results in GFP expression throughout the embryo (n = 43/43 GFP+). (C, D) Injection of the rhoua ATG MO represses the expression of GFP due to this construct (n = 0/57 GFP+). (A, C) Brightfield microscopy. (B, D) GFP fluorescence. (E) Gel electrophoresis image shows that the rhoua splice MO2 blocks the proper splicing of rhoua and results in five different alternative splice products (Band 1-5). (F) Diagram of the rhoua genomic structure shows how the five different rhoua alternative splice products were produced by the rhoua splice MO2 (green). Light blue boxes represent exons. Purple lines represent new splice isoforms created due to the splice morpholino. Red line represents the normal splicing between exon 1 and exon 2. |
|
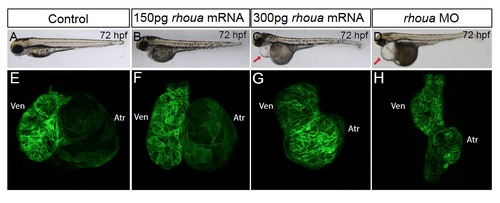
Injecting high doserhouaRNA results in enlarged and dysmorphic hearts. (A, B, E, F) Similar to (A, E) control Tg(cmlc2:ras-eGFP) embryos (n = 79), (B, F) injection of 150 pg of rhoua RNA into wild-type Tg(cmlc2:ras-eGFP) embryos did not cause any significant cardiac phenotypes (n = 0/33). However, (C, G) injection of 300 pg of rhoua resulted in larger and dysmorphic hearts (n = 55/77), which is in contrast to the smaller hearts observed in (D, H) rhoua MO injected embryos (n = 83/102). Arrow points to pericardial edema. |
Reprinted from Developmental Biology, 389, Dickover, M., Hegarty, J.M., Ly, K., Lopez, D., Yang, H., Zhang, R., Tedeschi, N., Hsiai, T.K., Chi, N.C., The atypical Rho GTPase, RhoU, regulates cell-adhesion molecules during cardiac morphogenesis, 182-91, Copyright (2014) with permission from Elsevier. Full text @ Dev. Biol.