- Title
-
Selective Histone Deacetylase 6 Inhibitors Restore Cone Photoreceptor Vision or Outer Segment Morphology in Zebrafish and Mouse Models of Retinal Blindness
- Authors
- Sundaramurthi, H., Roche, S.L., Grice, G.L., Moran, A., Dillion, E.T., Campiani, G., Nathan, J.A., Kennedy, B.N.
- Source
- Full text @ Front Cell Dev Biol
|
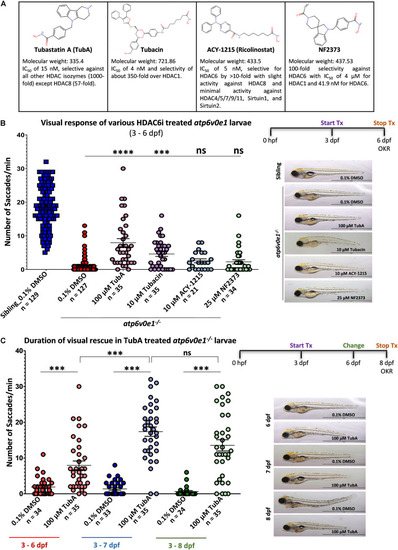
Selective HDAC6i significantly restores visual function in PHENOTYPE:
|
|
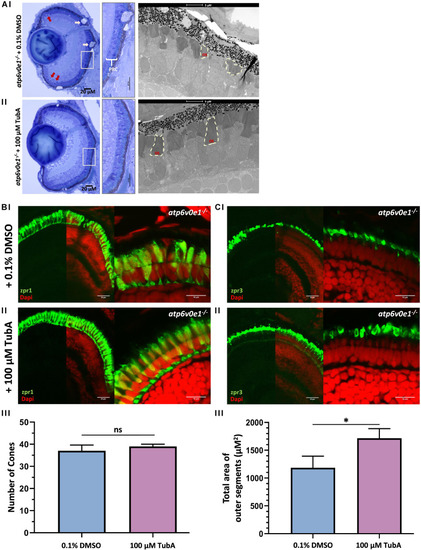
TubA treatment improved retinal morphology in PHENOTYPE:
|
|
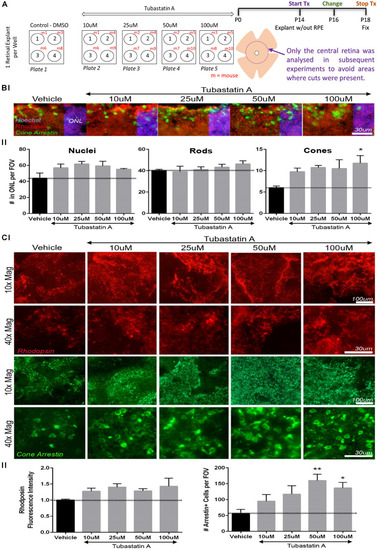
TubA preserves cone cells in |
|
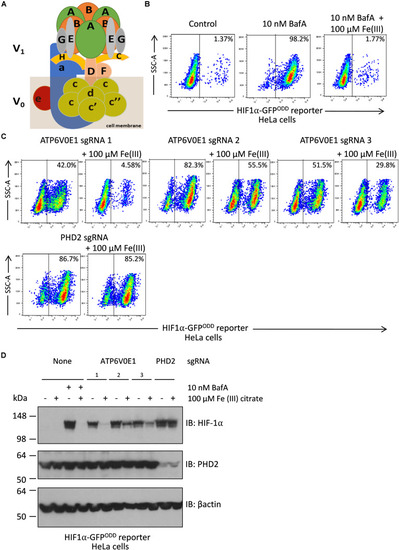
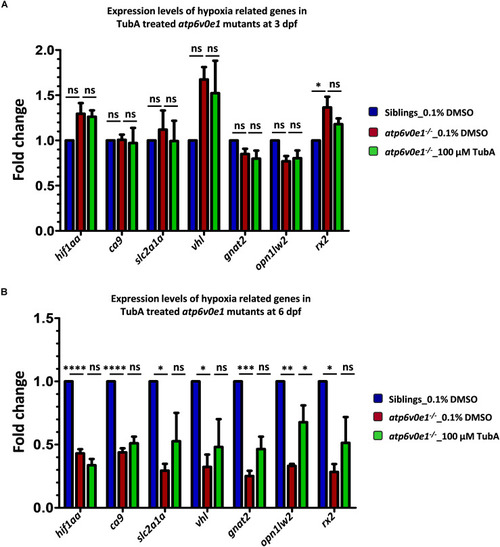
Iron supplementation restores HIF-1α levels to normal following |
|
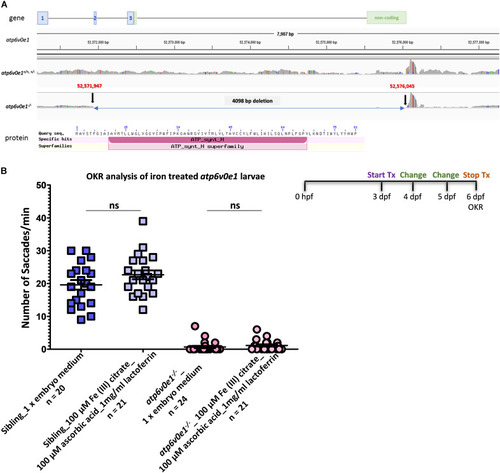
Iron supplementation did not restore vision in PHENOTYPE:
|
|
Analysis of EXPRESSION / LABELING:
PHENOTYPE:
|
|
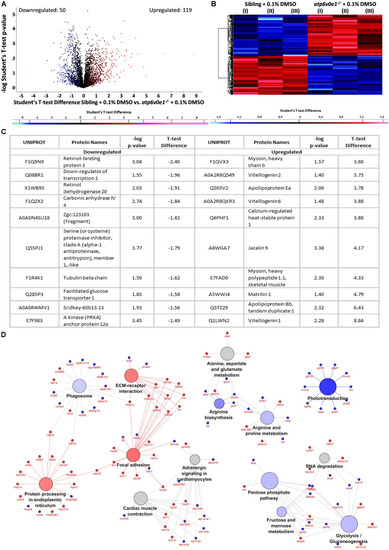
Multiple pathways are implicated in the disease pathomechanism of |
|
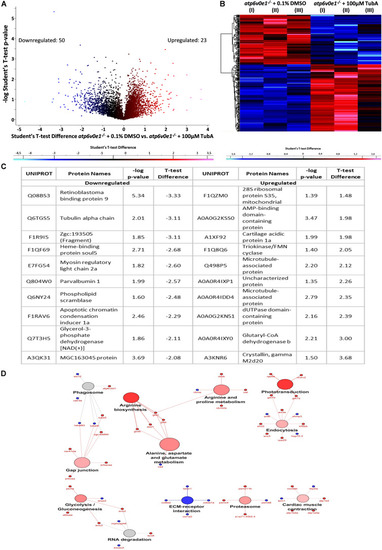
TubA plays a multifaceted role to improve visual function and preserve photoreceptors. |