- Title
-
Eyes shut homolog is required for maintaining the ciliary pocket and survival of photoreceptors in zebrafish
- Authors
- Yu, M., Liu, Y., Li, J., Natale, B.N., Cao, S., Wang, D., Amack, J.D., Hu, H.
- Source
- Full text @ Biol. Open
|
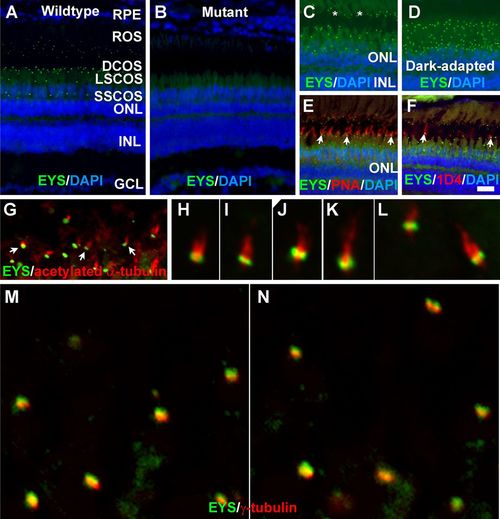
Zebrafish EYS protein was concentrated near the connecting cilium/transition zone. Zebrafish eye sections were immunostained with EYS antibody and PNA, and 1D4 antibody and antibodies against acetylated α-tubulin and γ-tubulin. The sections were counter stained with DAPI to show nuclei. (A) EYS antibody staining (green fluorescence) of wild-type retina (n=10). EYS immunoreactivity exhibited as a punctate pattern between the RPE and ONL. (B) EYS immunostaining of EYS-deficient retina (n=10). Note the absence of punctate fluorescence. (C,D) EYS immunostaining of light- and dark-adapted retinas. Note that some of the EYS punctate were located at the basal end of autofluorescence of rod outer segment (asterisks). The domain of EYS punctate shrank upon dark adaptation. (E,F) Double staining of EYS antibody with PNA and 1D4 antibody, respectively. Note that some of the EYS punctate were located on the basal end of the PNA-positive cone outer segment and 1D4-positive outer segment of long double cones (arrows). (G-L) Double staining of EYS (green) and acetylated α-tubulin (red). Note that EYS immunoreactivity is located on the basal end of acetylated α-tubulin. Arrows in G indicate examples at low magnification. (M-N) Double staining of EYS (green) and γ-tubulin (red) antibodies. Note that EYS is apposed to but apical of γ-tubulin. Scale bar in F: 20 µm for A-F. DCOS, double cone outer segment; GCL, ganglion cell layer; INL, inner nuclear layer; LSCOS, long single cone outer segment; ONL, outer nuclear layer; ROS, rod outer segment; RPE, retinal pigment epithelium; SSCOS, short single cone outer segment. EXPRESSION / LABELING:
|
|
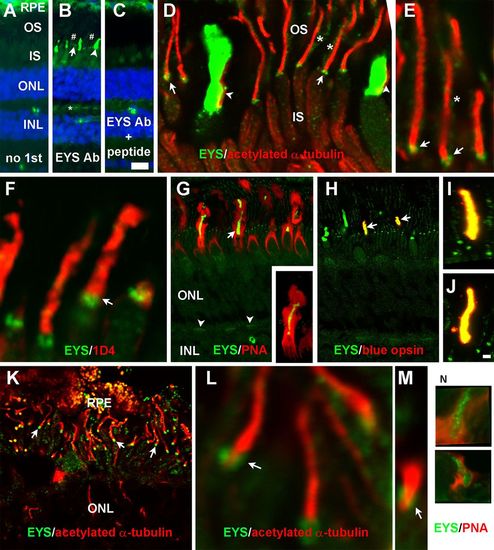
EYS protein in primate retina was concentrated not only at the CC/TZ but also in cone outer segments and was weakly expressed at rod outer segments and cone terminals. Eye cups from three 8-year-old female rhesus macaque monkey and a human donor of undisclosed age and sex were stained with antibodies against EYS, acetylated α-tubulin, rhodopsin, or blue opsin and PNA. (A-C) Staining with no primary antibody, EYS antibody, and EYS antibody and blocking peptide, respectively. Note EYS immunoreactivity was observed as punctate pattern (arrow), cone outer segment (arrowhead), and rod outer segment (# signs) between the RPE and ONL as well as cone terminals (asterisk). (D,E) Double immunostaining of EYS (green) and acetylated α-tubulin (red). Note EYS reactivity was located on the basal end of acetylated tubulin (arrows), rod outer segment (asterisks), and adjacent to cone axonemes (arrowheads). (F) Double staining of EYS antibody (green) with rhodopsin antibody 1D4 (red). EYS is concentrated in the position basal to 1D4 immunoreactivity (arrow) and some of the EYS reactivity overlapped with 1D4. (G) Double staining of EYS antibody (green) with PNA (red). Note that EYS immunoreactivity in the cone outer segment was completely wrapped by PNA-stained cone matrix. EYS immunoreactivity in the OPL was also reactive to PNA (arrowheads). Insert in G shows a single cone at higher magnification. (H-J) Double staining of EYS (green) and blue opsin (red) antibodies. Note that a subset of EYS-positive cone outer segment was immunoreactive to blue opsin antibody (arrows) and there is a complete overlap between EYS and blue opsin reactivities (I,J). (K-M) Double staining of EYS (green) and acetylated α-tubulin (red) of human retina. Note that EYS immunoreactivity was located on the basal end of acetylated α-tubulin immunoreactivity (arrows). (N) Some human EYS immunoreactivity was located within PNA-positive domain. Scale bar in C: 20 µm for A-C; scale bar in J: 2 μm for E,F,I,J,L-N; 5 μm for D; 10 μm for K; 20 μm for G,H. IS, inner segment; INL, inner nuclear layer; ONL, outer nuclear layer; OS, outer segment; PNA, peanut agglutinin; RPE, retinal pigment epithelium. |
|
EYS deficiency caused disruption of the ciliary pocket in cones. To determine the impact of EYS deficiency on structures near CC/TZ, we performed EM analysis on EYS-deficient zebrafish retina at 7, 40, 50, 80 dpf and 8 and 14 mpf. (A,B) Low and high magnification of same wild-type cone photoreceptor at 7 dpf. (C,D) Low and high magnification of the same EYS-deficient cone photoreceptor at 7 dpf. (E) Another example of EYS-deficient cone photoreceptor at 7 dpf. (F-H) Wild-type cone photoreceptors at 40 dpf. (I-L) EYS-deficient zebrafish cone photoreceptors at 40 dpf. (M) Wild-type rod photoreceptor at 40 dpf. (N,O) EYS-deficient rod photoreceptors at 40 dpf. (P) Wild-type rod photoreceptor at 8 mpf. (Q) EYS-deficient rod photoreceptor at 8 mpf. (R) Wild-type rod photoreceptor at 14 mpf. (S) EYS-deficient rod photoreceptor at 14 mpf. In wild type at 7 and 40 dpf cones, the ciliary pocket with electron dense lumen was clearly observed (B,F-H, arrows). The plasma membrane separating the outer and inner segments was in continuity with the membrane of the ciliary pocket (arrowheads in B,F-H). In EYS-deficient animals at 7 dpf, the cone ciliary pocket was normal except that the lumen of the mutant ciliary pocket was not as electron dense as the wild type (D,E, arrows). At 40 dpf, in EYS-deficient animals, the ciliary pocket in cone photoreceptors was collapsed (I and J, arrows) or was replaced with multiple membrane vesicles of electron density (K,L, arrowheads). The rod ciliary pocket in EYS-deficient zebrafish was largely maintained (M-O,Q,S, arrows). Scale bar in S: 100 nm for B,D-F,I,M-O; 200 nm for G,H,J,L,P-S. Ax, axoneme; BB, basal body; Mito, mitochondria; OS, outer segment. |
|
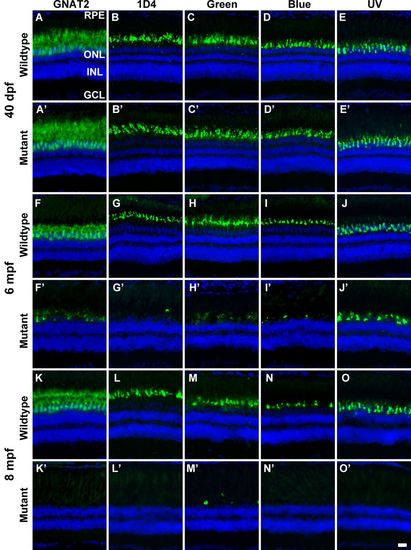
EYS-deficient zebrafish exhibited degeneration of cone photoreceptors. Zebrafish retinal sections were immunostained with GNAT2, 1D4, green opsin, blue opsin, and UV opsin antibodies. (A-E′) GNAT2, 1D4, green opsin, blue opsin, and UV opsin staining of 40 dpf wild-type and EYS-deficient zebrafish. There were no apparent differences between wild-type and EYS-deficient zebrafish. (F-J′) GNAT2, 1D4, green opsin, blue opsin, and UV opsin staining of 6 mpf wild-type and EYS-deficient zebrafish. Note GNAT2-, 1D4-, green opsin-, blue opsin-, and UV opsin-positive immunoreactivity was reduced in EYS-deficient zebrafish. (K-O′) GNAT2, 1D4, green opsin, blue opsin, and UV opsin staining of 8 mpf wild-type and EYS-deficient zebrafish. Note that GNAT2-, 1D4-, green opsin-, blue opsin-, and UV opsin-positive immunoreactivity was further reduced in EYS-deficient zebrafish. Scale bar: 20 µm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
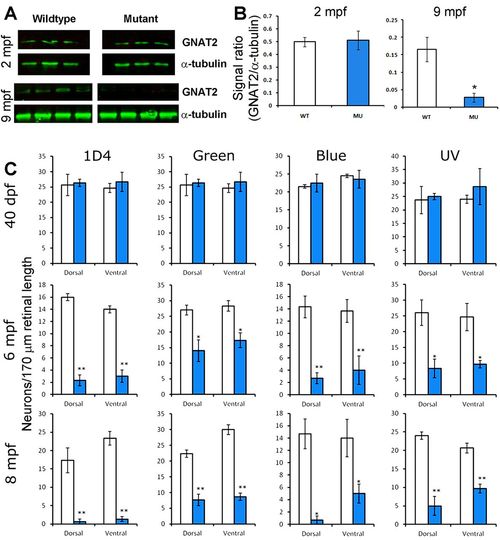
Reduction of GNAT2 protein and cone density in EYS-deficient zebrafish. Western blotting for GNAT2 was performed on retinal lysates from 2 mpf and 9 mpf zebrafish. Cone density was determined by counting 1D4-, green opsin-, blue opsin-, and UV opsin-positive cells in the dorsal and ventral retina. (A) Western blot for GNAT2 and α-tubulin. Note that while GNAT2 signal was not changed in 2 mpf mutant zebrafish, it was diminished in 9 mpf mutant zebrafish. (B) Quantification of western blot shown in A. *P<0.05, Student's t-test, two-tailed. Error bars indicate SEM (standard error of the mean). (C) Cone density in EYS-deficient zebrafish was normal at 40 dpf but reduced at 6 and 8 mpf. *P<0.05; **P<0.01 (repeated measures ANOVA). Error bars indicate s.e.m. EXPRESSION / LABELING:
PHENOTYPE:
|
|
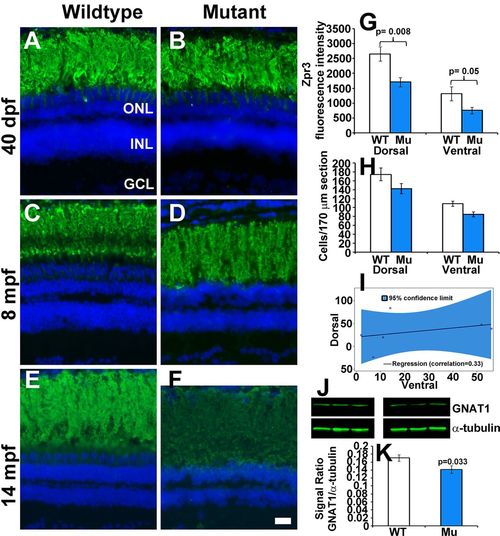
Rod photoreceptors degenerate in older EYS-deficient zebrafish. (A-F) Retinal sections were stained with Zpr3 and counter stained with DAPI. (A,B) Zpr3 staining of wild-type and EYS-deficient zebrafish at 40 dpf. (C,D) Zpr3 staining of wild-type and EYS-deficient zebrafish at 8 mpf. (E,F) Zpr3 staining of wild-type and EYS-deficient zebrafish at 14 mpf. (G) Quantification of Zpr3 immunofluorescence intensity of wild-type and EYS-deficient zebrafish at 14 mpf. (H,I) Rod quantification in wild-type and EYS-deficient zebrafish at 14 mpf. Rod cells in dorsal and ventral retina near the optic nerve heads were counted on H&E stained sections from sex- and body weight-matched animals. (J,K) GNAT1 western blot and quantification at 14 mpf. Western blot was carried out with GNAT1 and α-tubulin antibodies. Note that no significant differences were observed between wild-type and EYS-deficient zebrafish at 40 dpf and 8 mpf. However, Zpr3 immunoreactivity, rod cell numbers and GNAT1 protein were reduced in EYS-deficient zebrafish at 14 mpf. Scale bar for A-F: 20 µm. Error bars in G,H,K indicate s.e.m. EXPRESSION / LABELING:
PHENOTYPE:
|
|
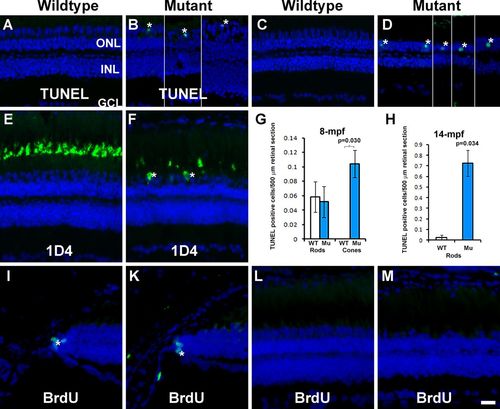
TUNEL-positive cells were observed in some cone and rod nuclei and BrdU incorporation were not changed in EYS-deficient retina. TUNEL staining and 1D4 immunostaining were carried out on retinal sections of 4, 8, and 14 mpf wild-type and EYS-deficient zebrafish retinas. BrdU-labeled nuclei were detected by immunofluorescence staining. (A) TUNEL staining of wild-type retina at 4 mpf. (B) TUNEL staining of EYS-deficient retina at 4 mpf. (C) TUNEL staining of wild-type retina at 14 mpf. (D) TUNEL staining of EYS-deficient retina at 14 mpf. (E) 1D4 immunostaining of wild-type retina. (F) 1D4 immunostaining of EYS-deficient retina. (G,H) Quantification of TUNEL-positive nuclei at 8 and 14 mpf, respectively. Error bars indicate s.e.m. (I,K) BrdU labeling at the ciliary margin of wild-type and EYS-deficient retina, respectively. (L,M) BrdU labeling at the central retina of wild-type and EYS-deficient retina, respectively. Note the presence of TUNEL-positive cone nuclei (asterisks in B), TUNEL-positive rod nuclei (asterisks in D), and 1D4 immunoreactive long double cone soma (asterisks in F) in EYS-deficient retinas. Also note absence of BrdU-labeled nuclei in the central retina of EYS-deficient animals. Scale bar: 20 µm. |
|
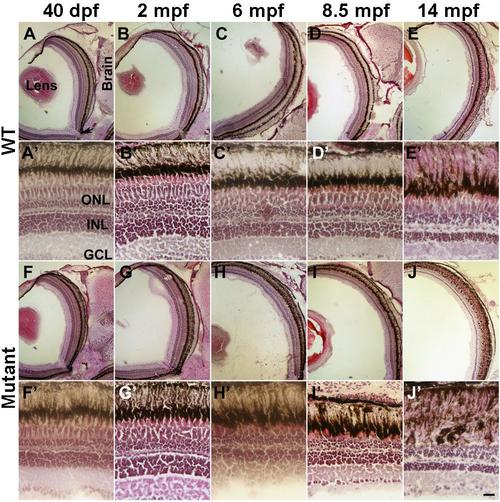
Degeneration of cone photoreceptors was revealed by H&E staining. Eye sections from 40 dpf and 2, 6, 8.5, and 14 mpf wildtype and EYS-deficient zebrafish were stained with H&E staining. (A-E) Low magnification images of wildtype animals. (A’-E’) High magnification images of wildtype animals. (F-J) Low magnification images of EYS-deficient animals. (F'-J') High magnification of EYS-deficient zebrafish. Loss of cones is apparent in EYS-deficient zebrafish at 6, 8.5, and 14 mpf. |