- Title
-
Tjp3/zo-3 is critical for epidermal barrier function in zebrafish embryos
- Authors
- Kiener, T.K., Selptsova-Friedrich, I., and Hunziker, W.
- Source
- Full text @ Dev. Biol.
|
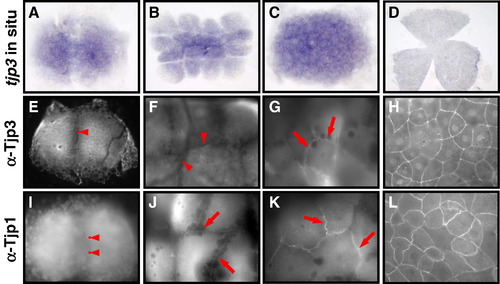
Tight junction biogenesis in the early zebrafish embryo. Tjp3/zo-3 whole mount in situ hybridization (A–D) and Tjp3/Zo-3 (E–H) and Tjp1/Zo-1 whole-mount immunofluorescence (I–L) of 2-cell stage/0.75 h (A, E, I); 8–16-cell stage/1.25 hpf (B, F, J); 64-cell stage/2 hpf (C, G, K); shield stage/6 hpf (D, H, L). Tjp3/zo-3 is a maternally supplied mRNA with in situ hybridization signals from the 2-cell stage (A–C). Expression is uniform up to 60% epiboly (D). Tjp1/Zo-1 and Tjp3/Zo-3 are already present in the 2-cell stage embryo, where they are localized diffusely in the cytoplasm but not at cell–cell contact sites (E, anti-Tjp3/Zo-3; I, anti-Tjp1, arrowheads). Tjp3/Zo-3 remains absent from cell–cell contact sites at the 8-cell stage (F, arrowheads) but Tjp1/Zo-1 already accumulates at cell–cell junctions (J, arrows). Tjp3/Zo-3 gets localized to cell–cell contacts only at the 64-cell stage (G, arrows), by a time when Tjp1/Zo-1 is strongly present in the junctional complex (K, arrow). By 6&nbs accumulates at cell–cell junctions (J, arrows). Tjp3/Zo-3 gets localized to cell–cell contacts only at the 64-cell stage (G, arrows), by a time when Tjp1/Zo-1 is strongly present in the junctional complex (K, arrow). By 6 hpf the enveloping cell layer contains Tjp3/Zo-3 and Tjp1/Zo-1 at all cell–cell junctions (G, H). |
|
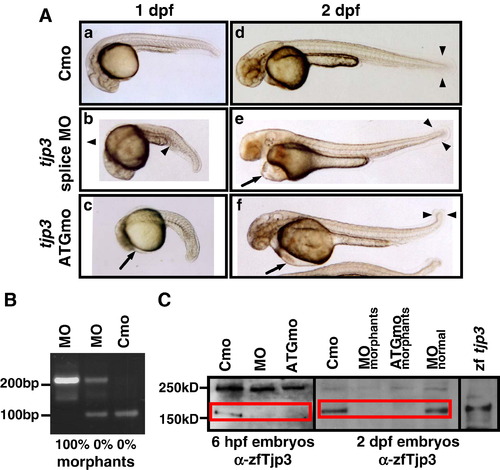
Characterization of the tjp3/zo-3 morpholino phenotype. Zebrafish embryos were injected with a control morpholino (Cmo), a splice site (MO) or a translation start morpholino (ATGmo) against tjp3/zo-3. (A) Both tjp3/zo-3 splice and translation start morpholino injected embryos present the same phenotypes. At 1 dpf embryos had shorter and curved tails with an expanded blood island (b, arrowhead). Some individuals showed an overall reduction in body length and the beginning of pericardial edema (c, arrow). By 2 dpf, pericardial edema was pronounced (e, f, arrows) and many individuals had no blood circulation. Morphants had curved tails and the size of the tailfin was reduced (e, f, arrowheads). (B) tjp3/zo-3 splice morpholino inhibits tjp3/zo-3 mRNA splicing. RT-PCR analysis was performed on embryos injected either with control morpholino (Cmo), or tjp3/zo-3 splice morpholino (MO) at 1 dpf. In control morpholino injected embryos, only one product of 97 bp corresponding to the properly spliced exon1/2 sequence was amplified (lane 3). In embryos injected with tjp3/zo-3 splice morpholino and selected for normal phenotypes, a 210 bp fragment containing the first intron sequence was amplified in addition to the 97 bp WT fragment (lane 2). Abnormal phenotypes were characterized by the absence of the 97 bp WT fragment and the presence of the 210 bp exon1/intron1/exon2 fragment (lane 1). (C) The tjp3/zo-3 splice and translation blocking morpholino reduce Tjp3/Zo-3 protein levels. Protein from whole 6 hpf (lanes 1-3) or 2 dpf (lanes 4-8) embryos was extracted and blotted with anti-zebrafish Tjp3/Zo-3 antibody. At 6 hpf, low levels of Tjp3/Zo-3 protein were detected in embryos injected with a control morpholino (Cmo, lane 1). No Tjp3/Zo-3 protein could be detected in embryos injected with tjp3/zo-3 splice morpholino (MO, lane 2) or tjp3/zo-3 translation blocking morpholino (ATGmo, lane 3). At 2 dpf both splice morpholino and ATG morpholino injected embryos were selected for morphant phenotypes. These individuals showed a strong reduction of Tjp3/Zo-3 protein levels (lanes 5 and 6) while splice morpholino injected embryos without phenotype had normal levels of Tjp3/Zo-3 protein (lane 7). WT embryos injected with Tjp3/Zo-3 expression vector served as a positive control (lane 8). |
|
The tjp3/zo-3 morpholino causes the loss of Tjp3/Zo-3 protein expression. Immunofluorescence of whole mount zebrafish at 1 dpf (A-D), 2 dpf (E-F), and cryosections at 6 dpf (G-H) stained with anti-Tjp3/Zo-3. Tjp3/Zo-3 protein is lost in morpholino-injected embryos from 1 dpf up to 6 dpf. The morphant phenotype correlates to the loss of Tjp3/Zo-3 since embryos selected for the lack of an apparent phenotype still express Tjp3/Zo-3 protein (data not shown). At 1 dpf there is a loss of Tjp3/Zo-3 labeling in the olfactory placodes (A, B, arrows), otic placodes (A, B arrowheads), and skin (C-F arrowheads). At later stages, Tjp3/Zo-3 protein is absent from the apical membranes of the pronephric ducts (E-H, arrows), and intestine (G, H, arrowheads). |
|
The tjp3/zo-3 morpholino effect is specific. Analysis of tjp3/zo-3 morpholino injected embryos at 2 dpf showed that the severity of the phenotype is dosage dependent and that the injection of splice-site morpholino and translation morpholino cause the same distribution of phenotypes. (A) Zebrafish embryos were injected at the 1-cell stage with 6 ng/1.5 nl control morpholino, 1.5 nl tjp3/zo-3 splice morpholino (MO) at different concentrations (2 ng, 6 ng, 12 ng) and 1 ng/1.5 nl tjp3/zo-3 translation blocking morpholino (ATGmo). At 2 dpf the phenotypes were assessed by morphological criteria. The death rate was between 20% (2 ng MO) and 40% (12 ng MO). The phenotypes were classified into 4 groups according to severity: no circulation (with edema and curved tail), edema (with or without curved tail), curved tail only, and reduced tailfin only. At a high dose of tjp3/zo-3 morpholino (12 ng) the major phenotype of the survivors was absent circulation with edema and a curved tail (55%), whereas at a low dose (2 ng) it was a reduction of the tailfin (20%) with less than 3% of edema. The injection of only 1 ng of translation blocking morpholino (ATGmo) resulted in a similar distribution of phenotypes as that of 12 ng splice morpholino (MO). Mean and S.E.M.; n ≥ 100 per experiment; 3–5 independent experiments. 2-way ANOVA; p < 0.05 (12 ng MO), p < 0.01 (1 ng ATGmo). Asterisks indicate statistical significance. (B) Phenotypes of injected embryos at 2 dpf. Control morpholino injected embryo (a); tjp3/zo-3 splice morpholino injected embryos (b, d); tjp3/zo-3 translation blocking morpholino injected embryos (c, e). Mild phenotypes presented with crooked tail buds and reduced tailfin size (b, c, arrowheads), while severely affected embryos had no circulation and often an accumulation of red blood cells (d, e, arrows). PHENOTYPE:
|
|
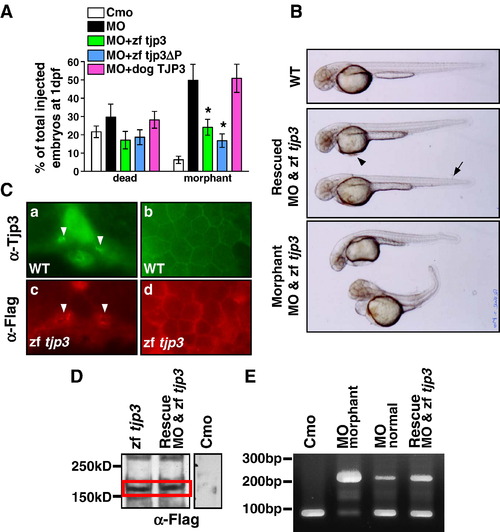
Rescue of the phenotype. Tjp3/zo-3 morphants can be rescued by full-length zebrafish tjp3/zo-3 and a truncated zebrafish tjp3/zo-3 without the C-terminal PDZ-binding motive, but not dog TJP3/ZO-3. (A) tjp3/zo-3 morphants can be rescued by co-injection of tjp3/zo-3 splice morpholino and pcDNA3.1 expression vector containing full-length Flag-tagged zebrafish tjp3/zo-3 (zf tjp3) or mutant zebrafish tjp3/zo-3 without the PDZ-binding motif (zf tjp3ΔP). Co-injection of pcDNA3.1 dog TJP3/ZO-3 cannot rescue the morphant phenotype, even tough the protein is expressed and enriched in membrane associated protein fractions of zebrafish (data not shown). Phenotypes were counted at 1 dpf. Mean and S.E.M.; n ≥ 100 per experiment; 2–6 independent experiments. 2-way ANOVA; p < 0.01. Asterisks indicate statistical significance. (B) Rescued embryos showed a straight body axis, little (arrowhead) or no edema, and only mild tailfin malformations (arrow). Non-rescued embryos presented with the morphant phenotype as described in Fig. 2 and Fig. 4. (C) Immunofluorescence on cryosections of WT embryos (a, b) and embryos injected with pcDNA3.1 Flag-tagged zebrafish tjp3/zo-3 (c, d) at 2 dpf. WT embryos were labeled with Tjp3/Zo-3 antibody to detect endogenous Tjp3/Zo-3 localization (a, b). Ectopically expressed Tjp3/Zo-3 localizes accurately. Anti-Flag staining was detected in the apical side of the pronephric ducts (c, arrowheads) and in the skin (d). (D) Anti-Flag Western blot at 2 dpf. Embryos were injected with control morpholino (Cmo), pcDNA3.1 zebrafish tjp3/zo-3 (zf tjp3), or co-injected with morpholino and pcDNA3.1 zebrafish tjp3/zo-3 (rescue). Ectopic Flag-tagged Tjp3/Zo-3 is expressed in embryos injected with tjp3/zo-3 alone (lane 1) as well as in the rescued embryos (lane 2). No Flag labeling was detected in controls (lane 3, different experiment). (E) RT-PCR analysis was performed using total RNA of 2 dpf embryos to check for the activity of the splice morpholino in rescued embryos. In control embryos (lane 1) and in morpholino injected embryos without phenotypes (lane 3), the properly spliced 97 bp wt band was amplified predominantly. However, tjp3/zo-3 morphants had a strong unspliced band at 210 bp (lane 2), which was also present in rescued embryos (lane 4). |
|
The loss of Tjp3/Zo-3 leads to a disruption of the TJ ultrastructure. (A) Cryosections of 6 hpf embryos stained with anti-Tjp3/Zo-3 (a, b) and anti-Tjp1/Zo-1 (c, d). Embryos were injected with control morpholino (a, c, Cmo) or tjp3/zo-3 splice morpholino (b, d, MO). Tjp3/Zo-3 protein is absent from most TJs in the EVL of morpholino injected embryos (b), but no phenotype is observed at that stage yet. The same embryos show a slight reduction in Tjp1/Zo-1 staining (d). (B) TEM of morpholino injected embryo sections show a marked loss and/or a disruption of the electron dense plaque of junctions in the EVL of morphants at 6 hpf. In control morpholino injected embryos, TJs can be observed at every cell–cell contact between neighboring cells of the EVL (Cmo, arrow). In the splice morpholino injected embryos, more than 40% of the EVL cell–cell contacts had discontinuities (blebbing) (MO, red arrows) and another 40% of contacts did not have any electron dense plaque (MO, arrowheads). Embryos injected with tjp3/zo-3 translation blocking morpholino had an even greater number of missing TJs (ATG, arrowheads). (C) Statistical analysis of TJ integrity as seen in TEM at 6 hpf. Two control and five morpholino injected embryos were analyzed. All contacts between adjacent EVL cells were classified into one of three groups. 1) Normal TJs, where cell membranes were in close contact throughout the length of the TJ, 2) discontinuous TJs with blebs, where cell membranes detached from each other, thus reducing the effective length of the TJ seal, and 3) junctions where no electron dense plaque was found between neighboring EVL cells. About 25% of TJs in control embryos had blebs (bar 1). In splice morpholino injected embryos, 47% of TJs showed blebbing and another 40% of the junctions were missing an electron dense plaque (bar 2). 62% of EVL cell–cell contacts in ATGmo injected embryos lacked an electron dense plaque (bar 3). N = 3 for Cmo, n = 3 for ATGmo, n = 5 for splice MO; number of cell–cell contacts counted per embryo 6–9; 2 independent experiments; t-test; p < 0.05. |
|
The asymmetric distribution of plasma membrane proteins is maintained in tjp3/zo-3 morphants. (A) Epithelial cell polarization in the EVL. Cryosections of control morpholino (a, c) and tjp3/zo-3 morpholino (b, d) injected embryos at 6 hpf. The EVL, a monolayer of epithelial cells that surrounds the blastoderm, is shown. EVL cells retain their elongated, flattened morphology in the morphants (b, d). These cells are still polarized with adherens junctions along their basolateral membranes, as shown by labeling with anti β-catenin (a, b) and anti pan-Cadherin (c, d) antibodies. Both markers remained restricted to the basolateral surfaces (lateral membranes, arrowheads; basal membranes, arrows). (B) Epithelial cell polarization in the pronephric ducts. Whole mount in situ hybridization of Pax2b (a, b); Immunofluorescence on cryosections: anti-Tjp3/Zo-3 (green) and anti-E-cadherin (red) (c, d); anti-PKCζ (green) and anti-Na+/K+-ATPase (red) (e, f). Control morpholino 1 dpf (a, c, e): anti-Tjp3/Zo-3 (green) and anti-E-cadherin (red) (c, d); anti-PKCζ (green) and anti-Na+/K; tjp3/zo-3 morpholino 1 dpf (b, d, f). In situ hybridization with Pax2b shows that the pronephric ducts extend normally in tjp3/zo-3 morphants (a, b, arrowheads). Morphants lost Tjp3/Zo-3 protein from the apical side of the pronephric ducts as shown above (c, d). E-cadherin staining of the basolateral membranes of the pronephric ducts is maintained in morphants (d, arrows). The polarized distribution of the basolateral Na+/K+-ATPase (e, f, arrows) and the apical PKCζ (e f, arrowheads) are also maintained in the pronephric duct. EXPRESSION / LABELING:
|
|
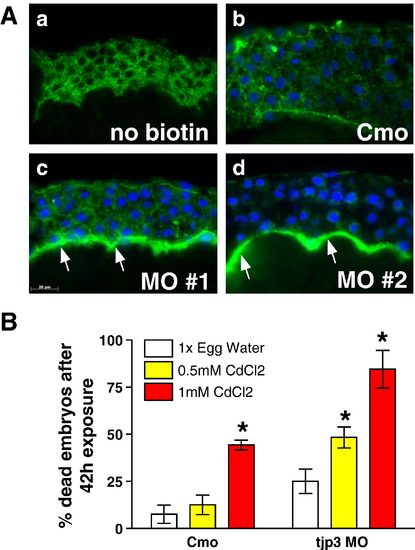
Tjp3/Zo-3 is essential for EVL permeability barrier function. (A) Biotin permeability assay: negative control without biotin (a), control morpholino (b) and tjp3/zo-3 morpholino (c, d) injected embryos were dechorionized at 6 hpf and exposed to 1 mg/ml Biotin in egg water. Cryosections were probed with labeled streptavidin and images taken using identical parameters. In Cmo injected embryos, although some background staining for streptavidin was observed (a), only little biotin diffusion was detected (b). In 40% of the tjp3/zo-3 morpholino injected embryos, a significant accumulation of biotin was observed in the segmentation cavity between the blastoderm and the yolk syncytial layer (c, d, arrows), demonstrating a compromised epidermal barrier in embryos lacking Tjp3/Zo-3. (B) Morphant embryos are more sensitive to the toxic heavy metal cadmium as compared to control embryos. Embryos injected with either control morpholino (Cmo) or tjp3/zo-3 splice morpholino (MO) were dechorionized at 6 hpf and grown in 1x egg water as a negative control or in egg water containing 0.5 mM CdCl2 or 1 mM CdCl2. Significance of differences was established using 2-way analysis of variance. Cmo embryos had a mortality of ∼ 20% after 42 h exposure to low toxicity CdCl2, whereas the mortality of morphants was significantly higher at ∼ 50% (p < 0.01). At the high cadmium toxicity, Cmo embryos had ∼ 50% mortality and mortality of MO injected embryos was again significantly higher at ∼ 80% (p < 0.05). Mean and standard error (S.E.M.); n ≥ 20 per experiment; 3–4 independent experiments. 2-way ANOVA; p < 0.01. Asterisks indicate statistical significance. |
|
Tjp3/zo-3 morphants are sensitive to osmotic stress. (A) The ability of tjp3/zo-3 morphants to survive high salt conditions is significantly reduced compared to control or rescued embryos. Embryos were either injected with control morpholino or tjp3/zo-3 splice morpholino or they were rescued by coinjecting MO with pcDNA3.1 containing zebrafish tjp3/zo-3 (MO & zf tjp3). Embryos were dechorionized and exposed to high salt conditions (see Materials and methods) at 6 hpf. Surviving embryos were counted at 1 dpf. A significant fraction of tjp3/zo-3 morpholino injected embryos died prematurely compared to control embryos. Mean and S.E.M.; n ≥ 20 per experiment; 3–5 independent experiments. t-test; p < 0.05. Asterisks indicate statistical significance. (B) Selection against tjp3/zo-3 morphants exposed to high salt or high magnesium during embryogenesis. High sodium and calcium salt concentrations reduced the survival of both normal (no phenotype) and morphant embryos. However, in 100x egg water and in 20x MgSO4 the number of morphants that survived to 2 dpf was significantly lower than that of normal embryos. Mean and S.E.M.; n ≥ 20 per experiment; 3–5 independent experiments. 2-way ANOVA; p < 0.05; statistical significance is indicated by asterisks. |
|
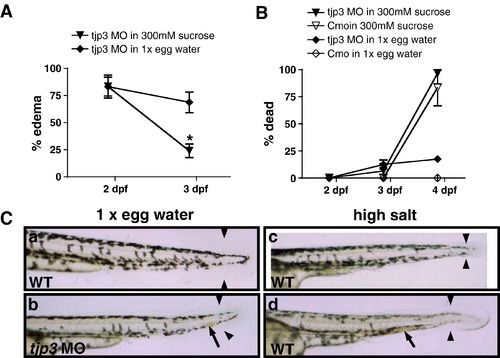
Compromised osmotic homeostasis mimics the tjp3/zo-3 morphant phenotype. (A) Pericardial edema of tjp3/zo-3 morphants can be reversed by growing the embryos in a hyperosmotic sucrose solution. At 2 dpf tjp3/zo-3 morphants were transferred into a 300 mM sucrose solution. ∼ 80% of tjp3/zo-3 morphants presented with edema (∼ 20% had a curved tail only). If grown in 1x egg water (diamonds), the incidence of edema only slightly decreased, due to individuals that died. However, if tjp3/zo-3 morphants were grown in 300 mM Sucrose (triangles), edema incidence dropped significantly. Mean and S.E.M.; n ≥ 10 per experiment; 5–6 independent experiments. 2-way ANOVA; p < 0.01. (B) Mortality of embryos grown in 300 mM sucrose. Morpholino injected embryos grown in 1x egg water had a mortality of only ∼ 20% at 4 dpf (diamonds), whereas the mortality for controls was very low (open diamonds). Placed in a hyperosmotic sucrose solution at 2 dpf, most morpholino injected (triangles) and control (open triangles) embryos survived up to 3 dpf, when edema incidence was counted. However, after 48 h exposure close to 90% of both control and morphant embryos died. There was no statistical significance between mortality of control or morphant embryos in 300 mM sucrose. Mean and S.E.M.; n ≥ 10 per experiment; 2–6 independent experiments. Two-way ANOVA; p > 0.5. (C) Exposure of WT embryos to high salt conditions mimics the tjp3/zo-3 morphant phenotype. WT embryos in 1x egg water have a straight tail, a wide tailfin and circulating blood (a). Tjp3/zo-3 morphants in 1x egg water presented with curved tail, reduced tailfin (b, arrowheads), the loss of blood circulation and an accumulation of red blood cells (b, arrow). 10–15% of control embryos raised in 100x egg water (c), 20x MgSO4 (d), 10x NaCl or 20x CaCl2 (data not shown), also presented with reduced tailfin (c, d, arrowheads), no blood circulation and accumulation of red blood cells (d, arrow). |
|
The asymmetric distribution of plasma membrane proteins is maintained in tjp3/zo-3 morphants. Epithelial cell polarization in the pronephric ducts. Immunofluorescence on cryosections: anti-Tjp3/Zo-3 (green) and anti-E-cadherin (red) (a-f); anti- PKCζ (green) and anti-Na+/K+-ATPase (red) (g-l). Control morpholino at 1 dpf (a, g) and 2 dpf (d, j); tjp3/zo-3 splice morpholino at 1 dpf (b, h) and 2 dpf (e, k) and tjp3/zo-3 translation blocking morpholino at 1 dpf (c, I) and 2 dpf (f, l). Note the strong reduction of Tjp3/Zo-3 protein from the apical side of the pronephric ducts in morphants (arrows), whereas E-cadherin or Na+/K+- ATPase staining on the basolateral membrane or PKCζ labeling on the apical membrane is not affected. |
|
Tjp3/Zo-3 is essential for EVL permeability barrier function. A. Biotin permeability assay: control morpholino (Cmo) and tjp3/zo-3 translation blocking morpholino (ATGmo) injected embryos were dechorionized at 6 hpf and exposed to 1 mg/ml Biotin in egg water. Cryosections were probed with labeled streptavidin and images taken using identical parameters. In Cmo injected embryos, although some background staining for streptavidin was observed, only little biotin diffusion was detected. In 6 out of 9 ATGmo injected embryos (correlating with the penetration rate of the morphant phenotpye), a significant accumulation of biotin was observed, in particular in the segmentation cavity between the blastoderm and the yolk syncythial layer, demonstrating a compromised epidermal barrier in embryos lacking Tjp3/Zo-3. |
Reprinted from Developmental Biology, 316(1), Kiener, T.K., Selptsova-Friedrich, I., and Hunziker, W., Tjp3/zo-3 is critical for epidermal barrier function in zebrafish embryos, 36-49, Copyright (2008) with permission from Elsevier. Full text @ Dev. Biol.