- Title
-
Pneumolysin-dependent and independent non-canonical autophagy processes mediate host defense against pneumococcal infection
- Authors
- Michno, B.J., Pooranachandran, N., Smith, T.C., Faught, E., Lipowská, S., Fenton, A.K., Meijer, A.H., Prajsnar, T.K.
- Source
- Full text @ Autophagy
|
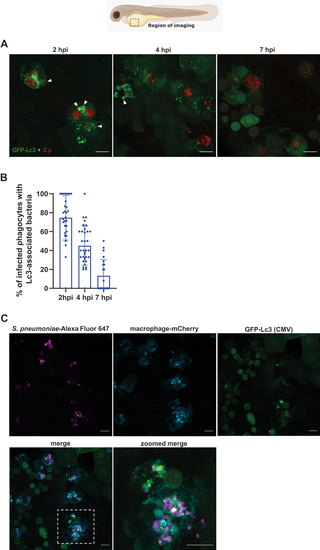
The autophagic response occurs during systemic infection with pneumococci. (A) Representative confocal images of |
|
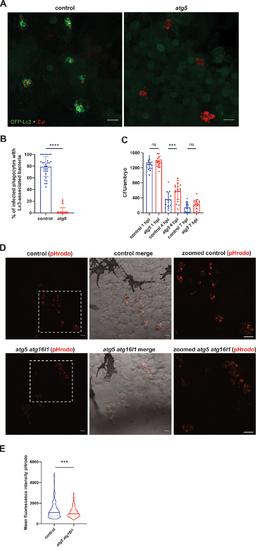
The autophagic response contributes to phagosomal acidification and pneumococcal clearance. (A) Representative confocal images of control (left panel) and |
|
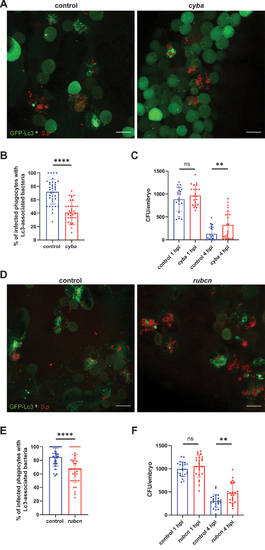
ROS-dependent autophagic response contribute to clearance of |
|
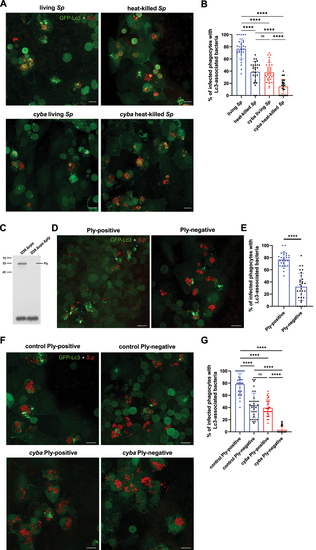
Live and pnemolysin-positive pneumococci are required to fully induce Lc3-mediated response within zebrafish macrophages. (A) Representative confocal images of |
|
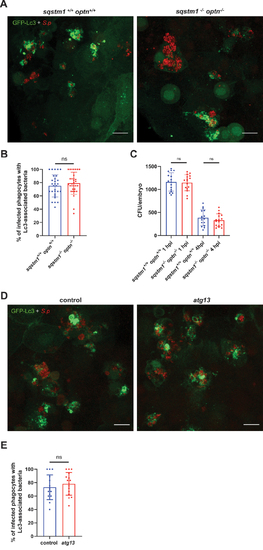
Sqstm1, optineurin and atg13 are not involved in autophagic response to |
|
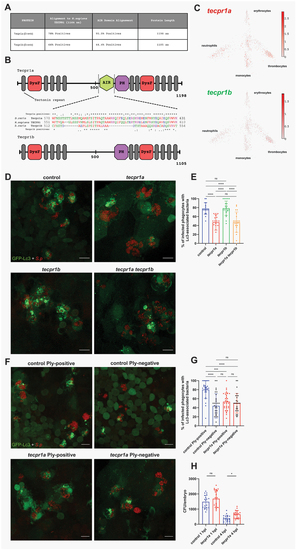
Tecpr1a, a likely ortholog of human TECPR1, controls ply-mediated autophagic response to pneumococci. (A) Table summarizing the alignment of zebrafish Tecpr1a and Tecpr1b proteins to the human TECPR1 protein. Columns display percentage positives (identical amino acids, as well as conserved or semiconserved substitutions) and length of the proteins. Alignments sequence similarities are shown for the full-length protein and the AIR domain, which exhibits the greatest divergence between the zebrafish paralogues (Tecpr1a and Tecpr1b). (B) Schematic representation of zebrafish Tecpr1a and Tecpr1b proteins, showing their domains: DysF – Dysferlin, AIR – ATG5-interacting region, and PH – Pleckstrin homology domain. Alignment of the AIR domain amino acid sequence of human TECPR1 and the zebfafish orthologs, Tecpr1a and Tecpr1b, is shown between the schematics. Symbols denote: (*) identical residues, (:) conserved substitutions, and (.) semiconserved substitutions. (C) Gene expression of adult zebrafish leukocytes determined using the zebrafish blood atlas [ |
|
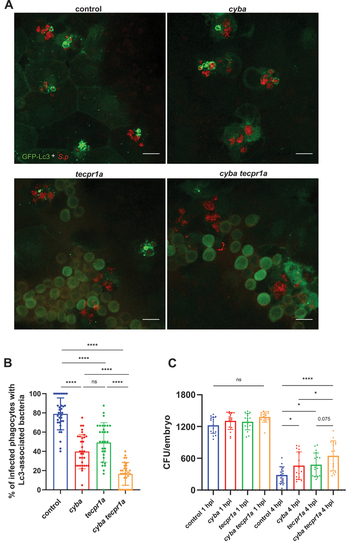
Both LAP and STIL are evoked in response to |
|
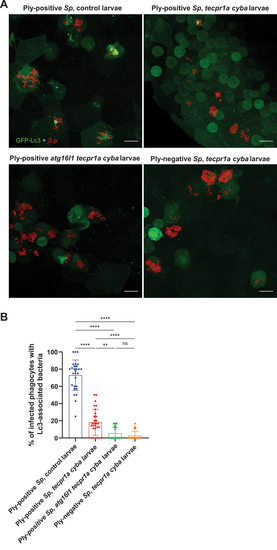
Involvement of ROS-independent LAP-like CASM of |
|
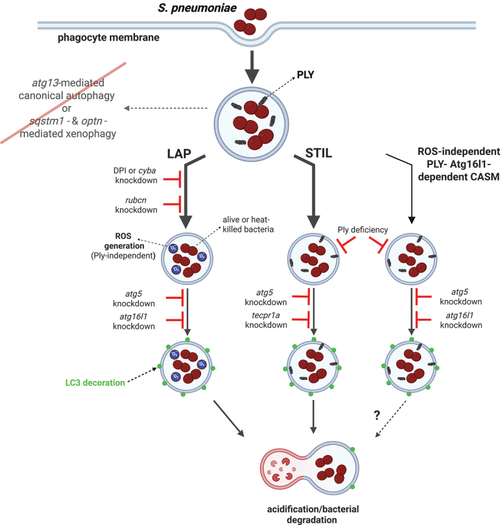
Model of autophagic response to pneumococci within zebrafish macrophages. After internalization by phagocytes, pneumococci are sequestered within Lc3-decorated vacuoles which are formed by three independent mechanisms: ROS-dependent LAP or two ROS-independent Ply-mediated pathways including STIL. LAP occurs as a result of osmotic imbalance within bacteria-containing phagosome caused by ROS production by NADPH oxidase. In contrast, STIL is induced upon membrane perturbation caused by secretion of bacterial pneumolysin manifested by sphingomyelin presence on the outside leaflet of the phagosome. STIL is dependent on Tecpr1a which recognizes sphingomyelin and mediates Lc3 lipidation. Last, the Atg16l1- and Ply-dependent pathway contributes to the smallest portion of Lc3 lipidation. However, the specific mechanisms controlling this process remain to be revealed. Following Lc3-assisted vacuolar sequestration, pneumococci are more efficiently targeted for lysosomal degradation and the Lc3 decoration is removed. The classical xenophagy receptors (sqstm1 and optineurin) as well as the canonical autophagy initiation molecule (atg13) are dispensable in this model. Despite the absence of these xenophagy-mediating factors, the bacteria are efficiently degraded. Figure created with BioRender. |