- Title
-
Amyloid-β Dysregulates Oligodendroglial Lineage Cell Dynamics and Myelination via PKC in the Zebrafish Spinal Cord
- Authors
- Balantzategi, U., Gaminde-Blasco, A., Kearns, C.A., Bayón-Cordero, L., Sánchez-Gómez, M.V., Zugaza, J.L., Appel, B., Alberdi, E.
- Source
- Full text @ Glia
|
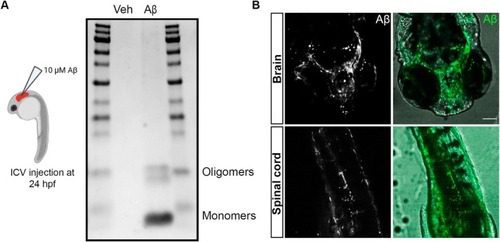
Intracerebroventricular Aβ injection into zebrafish larvae. (A) Schematic representation of the experimental approach, and the detection of Aβ species (monomers and oligomers) in the injection mixtures of vehicle and Aβ using Coomassie blue staining. (B) Representative images of fluorescent‐labeled Aβ diffusion into the brain and spinal cord of 24 hpf zebrafish larvae following ICV injection. Scale bar = 50 μm. |
|
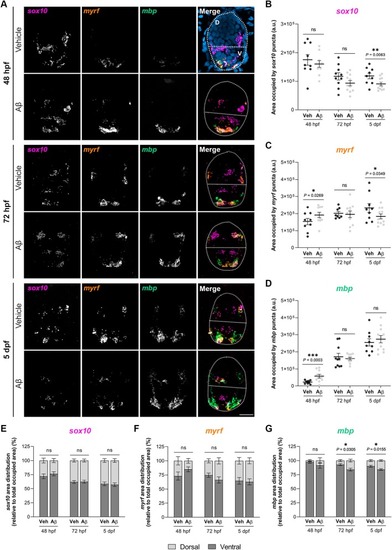
Aβ‐injected zebrafish larvae exhibit alterations in oligodendroglial lineage mRNA levels and regional distribution in the developing spinal cord. Zebrafish larvae were injected with Aβ or its vehicle at 24 hpf, and FISH assays were performed for |
|
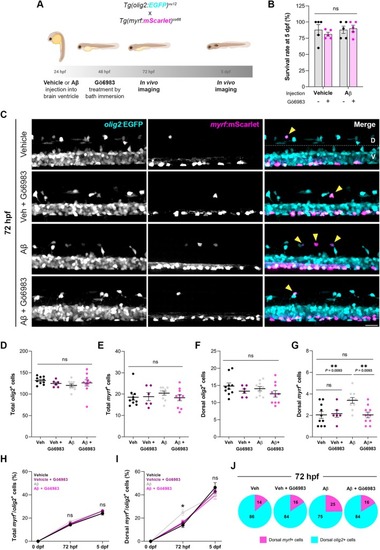
Aβ induces early oligodendrocyte differentiation through PKC, without affecting total cell numbers. (A) Transgenic zebrafish stably expressing |
|
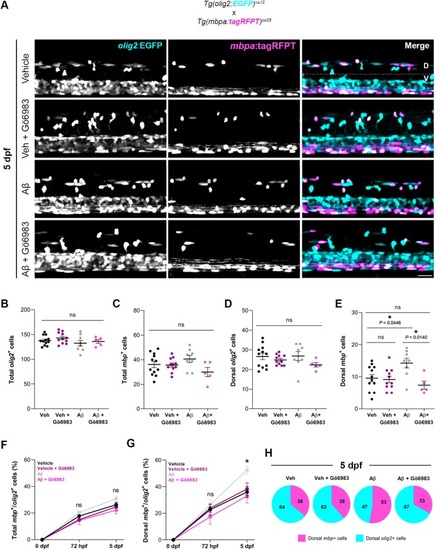
Aβ promotes oligodendrocyte maturation via PKC, without changing total cell numbers. (A) Representative lateral images of the spinal cord of live transgenic larvae stably expressing |
|
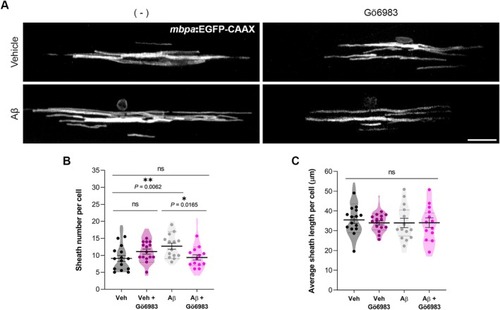
Aβ dysregulates dorsal myelin sheath number per oligodendrocyte via PKC activation. The |
|
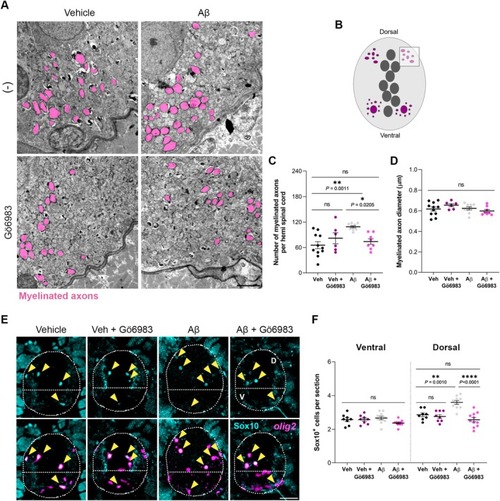
Aβ increases the number of dorsal oligodendroglial lineage cells and myelinated axons through PKC. Zebrafish larvae were intracerebroventricularly injected with Aβ (10 μM) or its vehicle at 24 hpf, and some were exposed to Gö6983 (500 nM) at 48 hpf. Larvae were fixed for electron microscopy or immunohistochemistry at 8 dpf. (A) Representative electron micrographs of the dorsal spinal cord of 8 dpf zebrafish larvae, with myelinated axons shaded in pink. (B) Schematic illustration of a cross‐section of the zebrafish spinal cord. The square represents the analyzed area. Histograms showing the (C) number and (D) diameter of myelinated axons in the dorsal area for each condition. Scale bar = 2 μm. |