- Title
-
Corticotropin-releasing hormone receptor 1 mediates the enhanced locomotor activity and metabolic demands to an acute thermal stress in adult zebrafish
- Authors
- Shvartsburd, Z., Vijayan, M.M.
- Source
- Full text @ J. Neuroendocrinol.
|
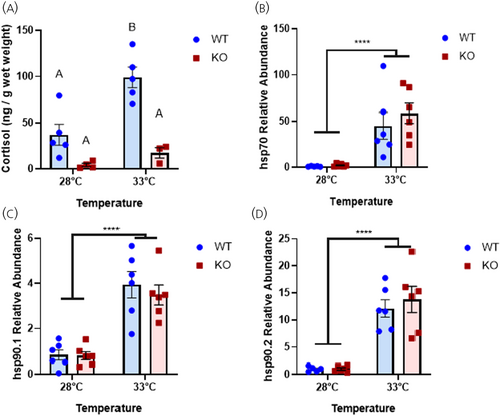
Lack of CRHR1 affects the thermal stress-mediated cortisol response but not the cellular heat shock response. (A) Whole-body cortisol concentration of WT and crhr1−/− zebrafish sampled from the housing system at 28°C or after 90 min exposure to the intermittent flow respirometer at 33°C using an in-house cortisol ELISA. (B) Transcript abundance of hsp70, (C) hsp90.1, and (D) hsp90.2 transcript abundance, measured from epaxial muscle of WT and crhr1−/− zebrafish exposed to a novel tank environment containing 28°C or 33°C water for 1 h. Transcripts were normalized to β-Actin expression and WT 28°C fish were standardized to 1 using the 2−ΔΔCt method. (A) Different letters denote significant difference (Two-way ANOVA, Holm–Sidak post hoc test, p < 0.05). (B–D) Asterisks denote significant difference (Two-way ANOVA, Holm–Sidak post hoc test, p < 0.05). Crhr1, corticotropin-releasing hormone receptor 1; ELISA, enzyme-linked immunosorbent assay; hsp, heat shock protein; KO, crhr1-/-; WT, wildtype. |
|
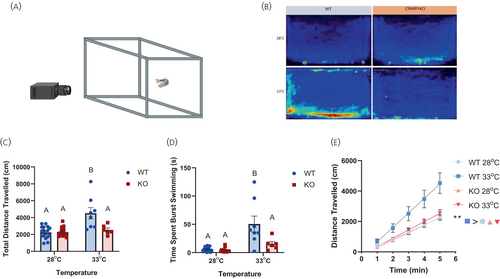
Lack of Crhr1 inhibits thermal stress-mediated zebrafish locomotory activity. (A) Diagram of the experimental setup for the vertical swim assessment. The recording camera was positioned to record from the side of the novel tank with the zebrafish. (B) Representative heatmap of the mean position of each treatment in the novel tank environment during the 5 min recording period, as observed by the camera field of view shown in (A). The light blue (less activity), yellow (more activity) and red (most activity) regions of the heatmap represent the overall activity of the fish in the tanks. (C) Total distance travelled by WT and crhr1−/− zebrafish exposed to a novel tank environment at 28°C or 33°C for 5 mins. (D) Total time zebrafish spent burst swimming, defined as swimming speeds greater than 90% of the average speed of each fish during the 5 min recording period. (E) Distance travelled by zebrafish per minute over the 5 min period. Adult zebrafish were transferred from their housing system to a novel tank containing either 28°C or 33°C system water. Video recording started immediately after placement of fish and lasted for 5 min. Behaviour was analysed using Noldus Ethovision XT 14. (B–D) Bars with different letters denote significant difference (Two-way ANOVA, Holm–Sidak post hoc test, p < 0.05). (E) Asterisks denote significant difference between treatment groups (linear regression, **p < 0.01). Crhr1, corticotropin-releasing hormone receptor 1; KO, crhr1-/-; WT, wildtype. PHENOTYPE:
|
|
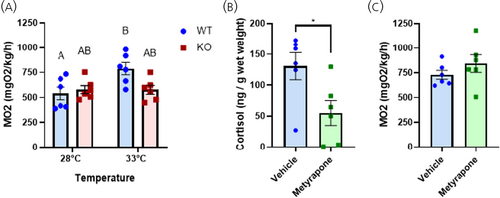
Lack of Crhr1 inhibits thermal stress-mediated metabolic rate changes in zebrafish. (A) Metabolic rate of WT and crhr1−/− fish at 28°C and 33°C after 60 min of acclimation in the resting intermittent-flow respirometer. MO2 was calculated as the mean of the three lowest consecutive measurements from each individual. (B) Whole-body cortisol concentration of WT zebrafish exposed for 4 days to 325.4 μM metyrapone and sampled immediately after 90 min exposure to the resting intermittent-flow respirometer at 33°C. (C) Metabolic rate of untreated and metyrapone-treated WT fish at 33°C after 60 min of acclimation in the resting intermittent-flow respirometer. MO2 was calculated as in (A). (A) Different letters denote significant changes between treatment groups (Two-way ANOVA, Holm–Sidak post hoc test, p < 0.05). (B, C) Asterisks indicate significant difference (Student's t-test; *p < 0.05). Crhr1, corticotropin-releasing hormone receptor 1; KO, crhr1-/-; MO2, metabolic rate; WT, wildtype. PHENOTYPE:
|
|
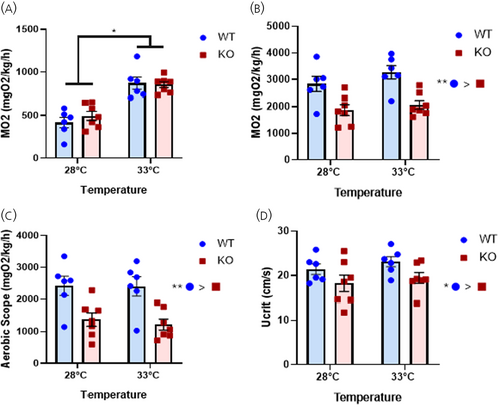
Lack of Crhr1 affects the thermal stress-mediated swimming performance. (A) The SMR of WT and crhr1−/− fish measured in a swim tunnel respirometer during the 1-h acclimation at either 28°C or 33°C before the forced swim. This value was taken as the single lowest MO2 measurement during acclimation at a water flow speed of 4.5 cm/s. (B) MMR of fish taken as the single largest MO2 value measured during the forced swim test. (C) Aerobic scope of fish calculated as the MMR − SMR. (D) Ucrit of fish following the forced swim test, where water flow was slowly increased by 4.5 cm/s every 10 min until the fish collapsed against the back grate of the swim tunnel. Asterisks indicate significant main effects (Two-way ANOVA, *p < 0.05, **p < 0.01). Crhr1, corticotropin-releasing hormone receptor 1; KO, crhr1−/−; MMR, maximum metabolic rate; MO2, metabolic rate; SMR, standard metabolic rate; Ucrit, critical swimming speed; WT, wildtype. PHENOTYPE:
|
|
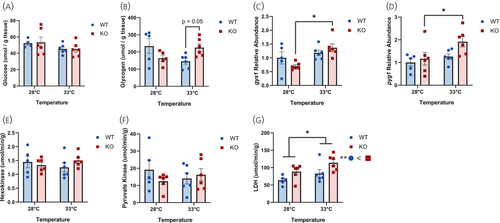
Lack of Crhr1 affects thermal stress-mediated muscle energy metabolism. (A) Glucose concentration and (B) glycogen concentration measured in WT and crhr1−/− zebrafish epaxial muscle exposed to the novel tank test for 1 h at either 28°C or 33°C. (C) Transcript abundance of muscle gys1 and (D) pyg1, and (E) enzyme activity of hexokinase, (F) pyruvate kinase, and (G) lactate dehydrogenase measured in WT and crhr1−/− zebrafish epaxial muscle exposed to the novel tank test for 1 h at either 28°C or 33°C. Asterisks indicate a significant difference between treatment interaction (Two-way ANOVA, Holm–Sidak post hoc, *p < 0.05) or (G) main effects (Two-way ANOVA, *p < 0.05, **p < 0.01). Crhr1, corticotropin-releasing hormone receptor 1; gys1, glycogen synthase 1; KO, crhr1−/−; LDH, lactate dehydrogenase; pyg1, glycogen phosphorylase 1; WT, wildtype. PHENOTYPE:
|