- Title
-
Differential Localization and Functional Roles of mGluR6 Paralogs in Zebrafish Retina
- Authors
- Haug, M., Haddad-Velioglu, S.A., Berger, M., Enz, A., Zang, J., Neuhauss, S.C.F.
- Source
- Full text @ Invest. Ophthalmol. Vis. Sci.
|
Localization of EXPRESSION / LABELING:
|
|
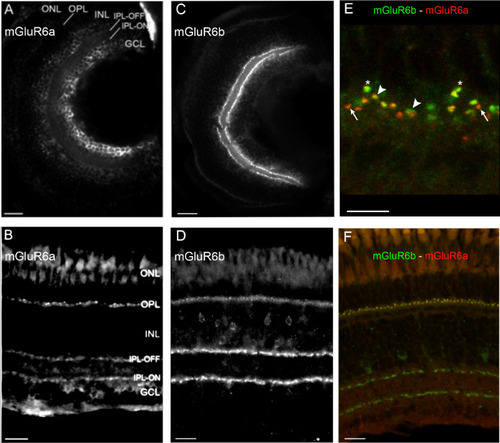
Retinal expression of mGluR6 paralogs in larval and adult zebrafish. ( |
|
mGluR6a paralog expression in photoreceptor subtype synapses. ( EXPRESSION / LABELING:
|
|
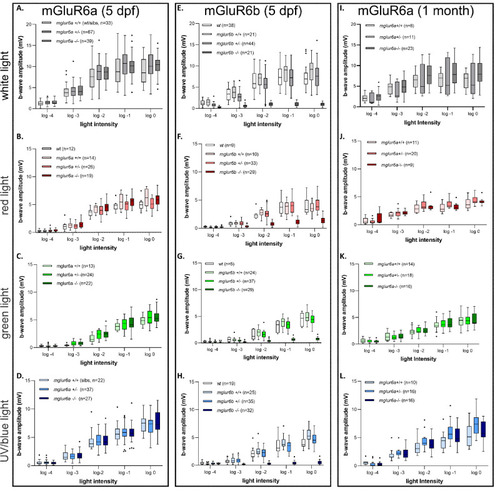
Spectral ERG measurements of the peak b-wave response in PHENOTYPE:
|
|
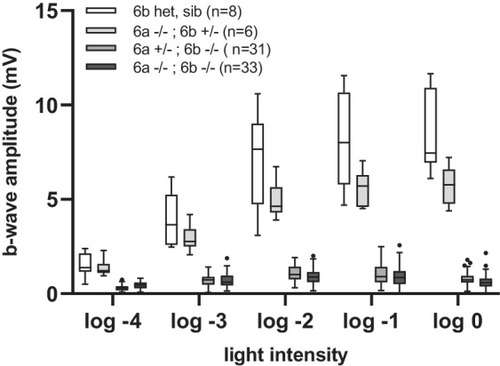
White light ERG measurements of the peak b-wave response in PHENOTYPE:
|