- Title
-
CRISPR/Cas9-Mediated fech Knockout Zebrafish: Unraveling the Pathogenesis of Erythropoietic Protoporphyria and Facilitating Drug Screening
- Authors
- Wijerathna, H.M.S.M., Shanaka, K.A.S.N., Raguvaran, S.S., Jayamali, B.P.M.V., Kim, S.H., Kim, M.J., Jung, S., Lee, J.
- Source
- Full text @ Int. J. Mol. Sci.
|
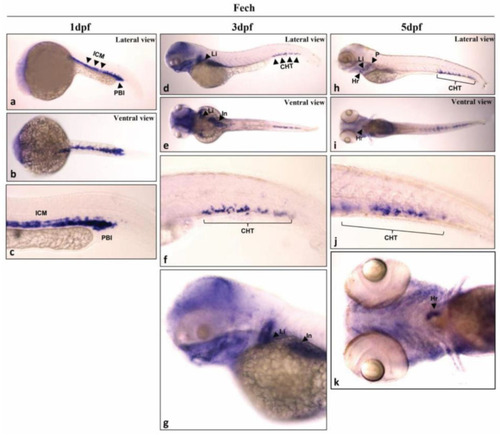
Spatial distribution of |
|
Tissue-specific expression of |
|
Generation and phenotyping of |
|
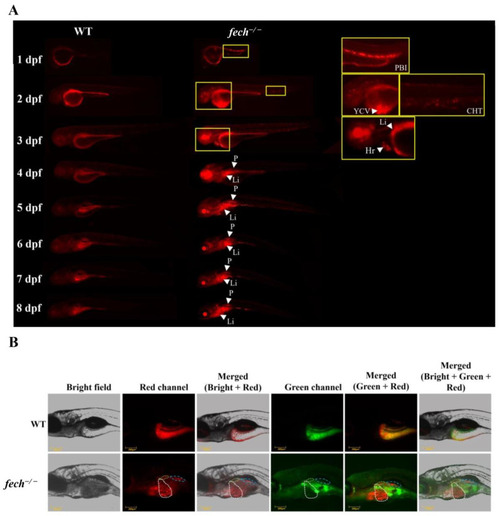
PPIX accumulation in |
|
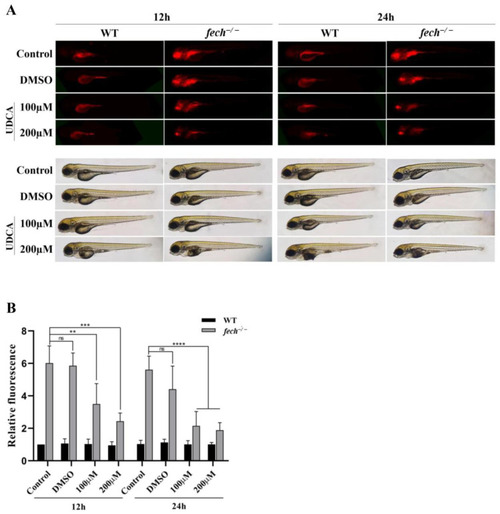
Reduction in PPIX accumulation in |
|
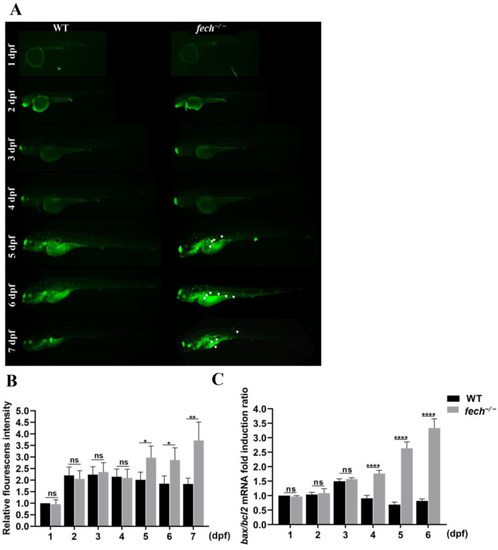
Apoptosis activation in |
|
Effect of UDCA treatment on apoptosis in |
|
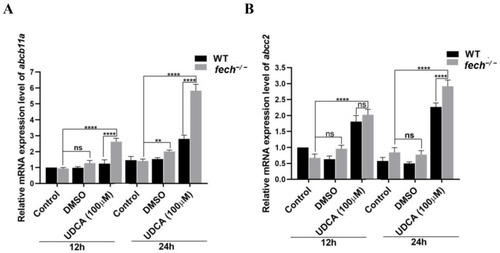
Effect of UDCA treatment on the expression of bile transporters in WT and |
|
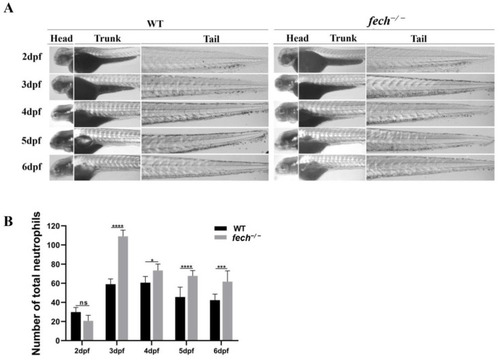
Temporal neutrophil production in |
|
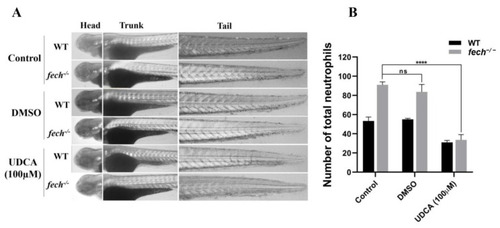
Amelioration of neutrophil accumulation by UDCA treatment in |
|
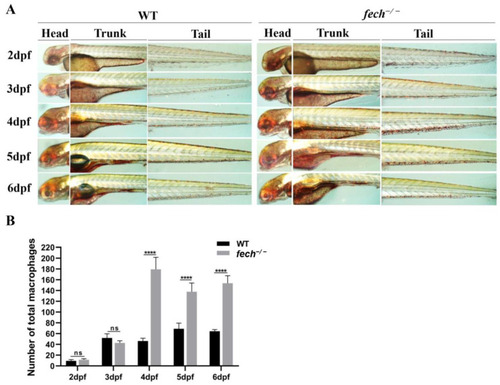
Changes in temporal macrophage production in |
|
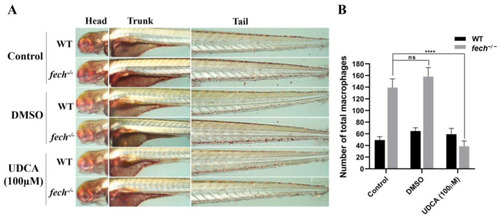
Attenuation of macrophage accumulation by UDCA treatment in |