- Title
-
Functional and Genetic Analyses Unveil the Implication of CDC27 in Hemifacial Microsomia
- Authors
- Song, W., Xia, X., Fan, Y., Zhang, B., Chen, X.
- Source
- Full text @ Int. J. Mol. Sci.
|
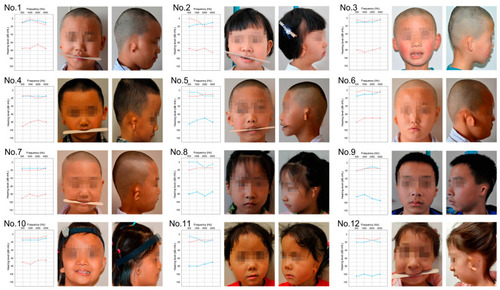
Phenotypic presentation and audiometry findings in 12 probands with hemifacial microsomia (HFM). The characteristic unilateral microtia (classified as Marx grading grade III) and the asymmetrical development of the mandible were presented in all probands, coupled with severe conductive hearing loss on the same side as assessed by pure tone audiometry. The blue line denotes the hearing threshold of the left ear, while the red line represents that of the right ear. |
|
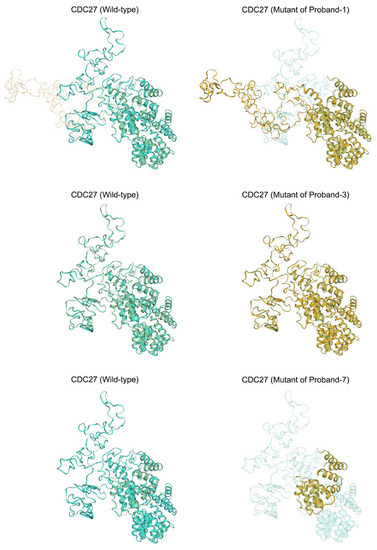
Comparative analysis of predicted tertiary structures in wild-type and mutant protein sequences near mutation sites. This figure presents structural superposition analyses to illustrate the alterations in protein structure resulting from the variants found in Proband I, Proband III, and Proband VII. Significant changes in the tertiary structure of the proteins due to the mutations were found in Proband I and Proband VII. In contrast, the predicted tertiary structure of the protein sequences flanking the variants from Proband III showed no discernible differences when compared to the wild-type structure, indicating that these particular mutations may not significantly alter the protein’s tertiary conformation. |
|
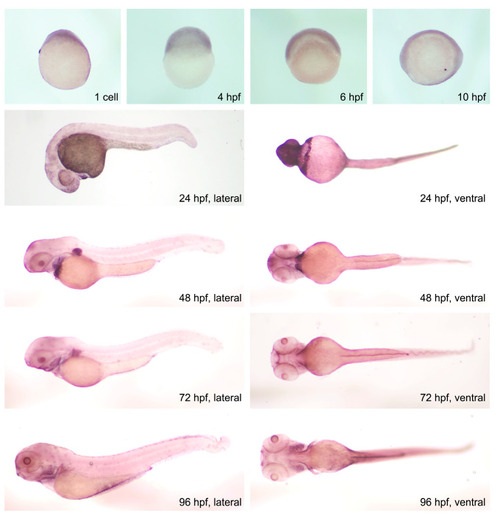
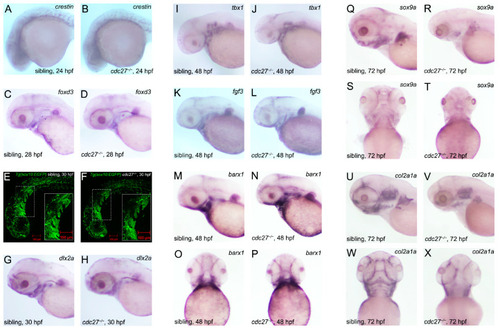
Temporal and spatial expression patterns of |
|
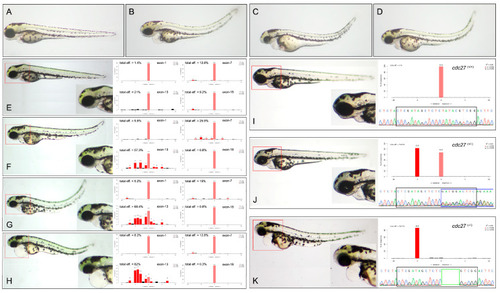
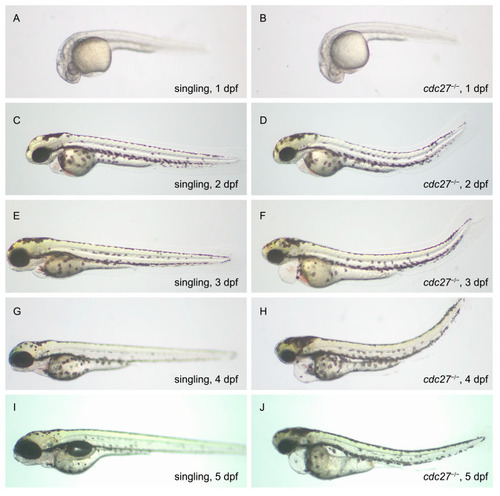
Comparison of phenotypes in wild-type and |
|
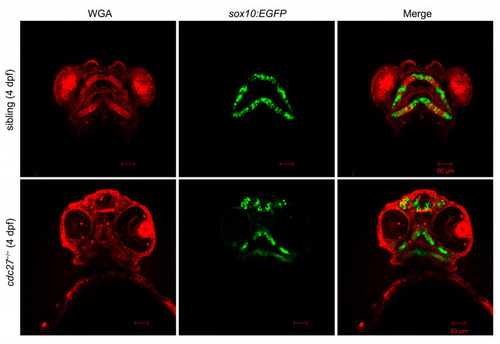
Phenotypic comparison between |
|
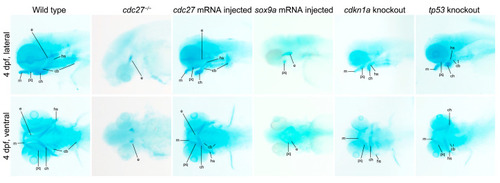
Alcian blue staining of pharyngeal arch cartilages in zebrafish embryos. This figure presents the staining results of pharyngeal arch cartilages using Alcian blue in various groups: wild-type, |
|
Comparative analysis of chondrocyte morphology in |
|
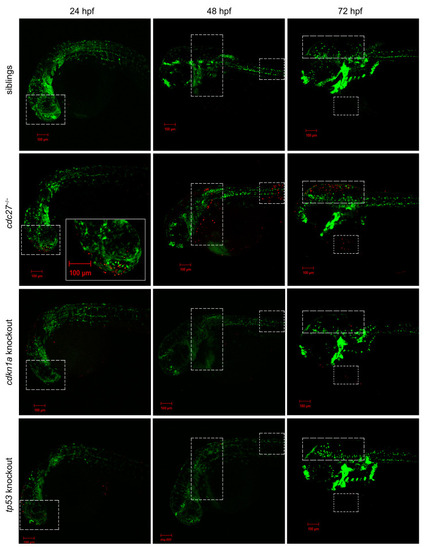
Investigating the impact of |
|
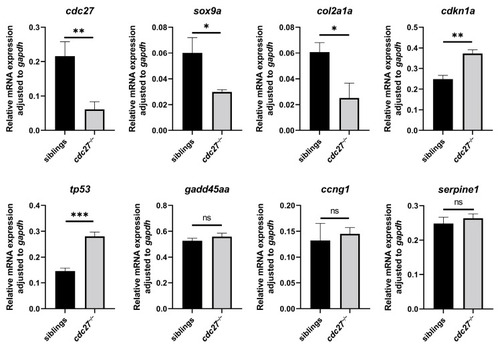
RT-qPCR analysis of gene expression in |
|
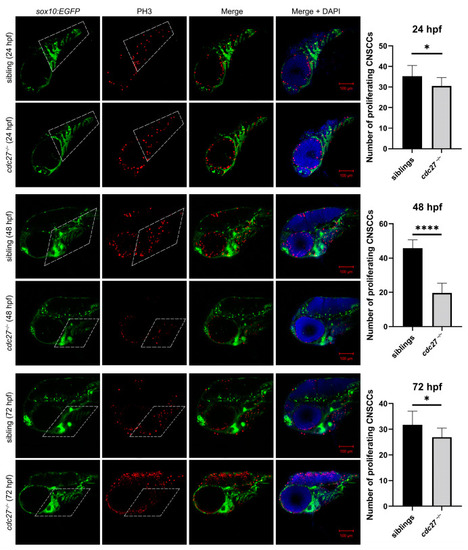
Comparison of CNCC proliferation in |
|
Analysis of somatic cell apoptosis in wild-type, |
|
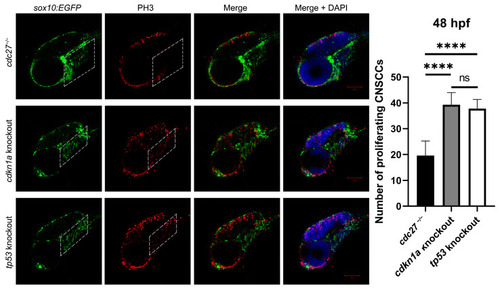
Comparative analysis of CNCC proliferation in |