- Title
-
Importin 13-dependent axon diameter growth regulates conduction speeds along myelinated CNS axons
- Authors
- Bin, J.M., Suminaite, D., Benito-Kwiecinski, S.K., Kegel, L., Rubio-Brotons, M., Early, J.J., Soong, D., Livesey, M.R., Poole, R.J., Lyons, D.A.
- Source
- Full text @ Nat. Commun.
|
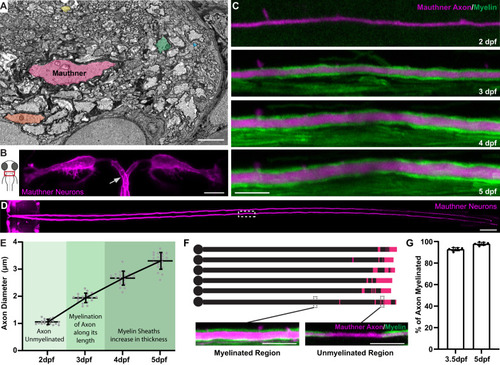
Zebrafish as a model to study axon diameter. |
|
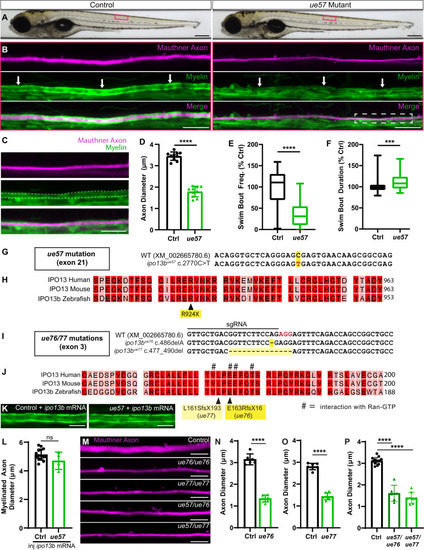
Identification of importin 13b mutants with reduced axon diameter growth. |
|
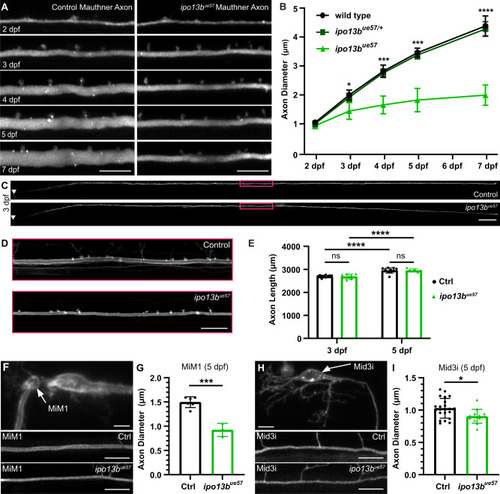
Live-imaging of axon diameter growth defects in importin 13b mutants. |
|
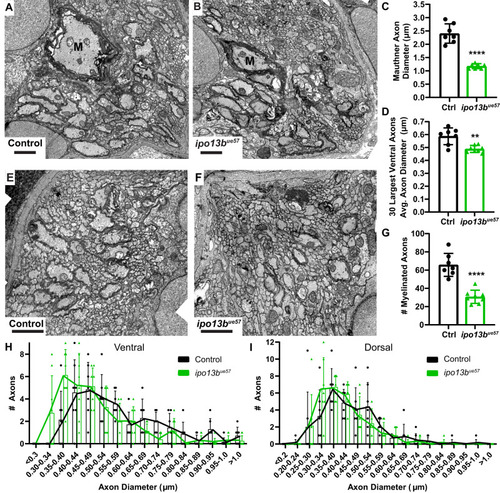
Electron microscopy of axon diameter growth defects in importin 13b mutants. |
|
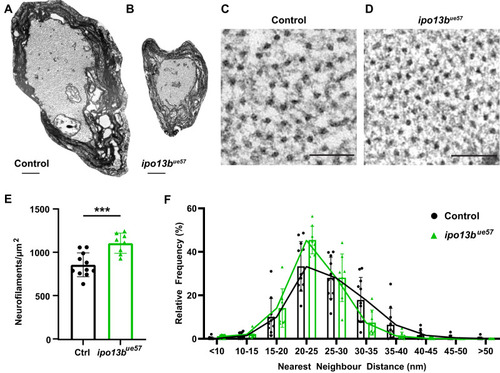
Disruption of importin 13b function increases neurofilament density in the Mauthner axon. |
|
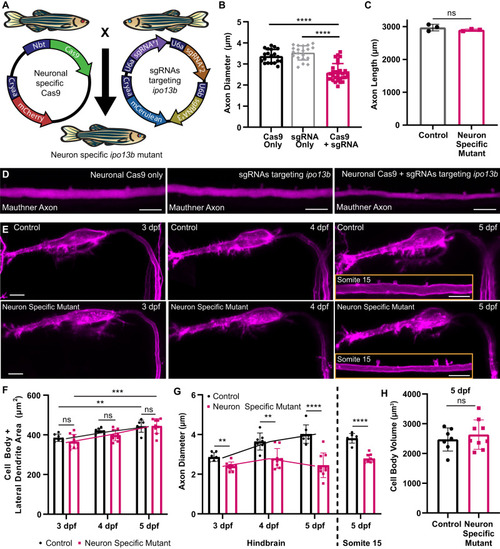
Disruption of axon diameter in neuron-specific |
|
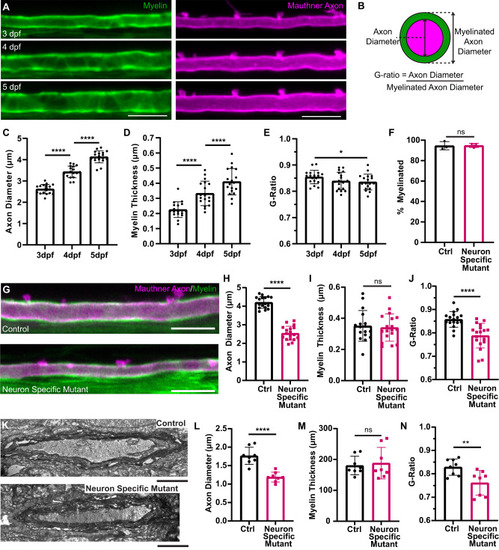
Reduced axon diameter growth does not change myelin thickness along the Mauthner axon. |
|
Diameter growth drives changes to conduction speeds along myelinated axons over time. |