- Title
-
The AMPK-Sirtuin 1-YAP axis is regulated by fluid flow intensity and controls autophagy flux in kidney epithelial cells
- Authors
- Claude-Taupin, A., Isnard, P., Bagattin, A., Kuperwasser, N., Roccio, F., Ruscica, B., Goudin, N., Garfa-Traoré, M., Regnier, A., Turinsky, L., Burtin, M., Foretz, M., Pontoglio, M., Morel, E., Viollet, B., Terzi, F., Codogno, P., Dupont, N.
- Source
- Full text @ Nat. Commun.
|
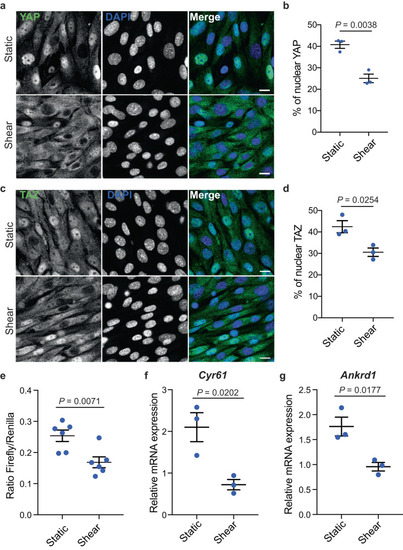
Physiological shear stress inhibits YAP/TAZ. |
|
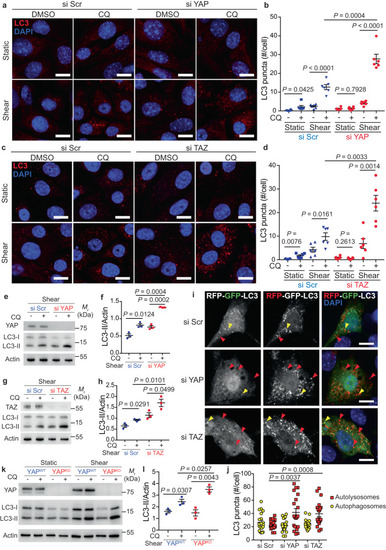
The loss of YAP or TAZ stimulates the autophagy flux during shear stress. |
|
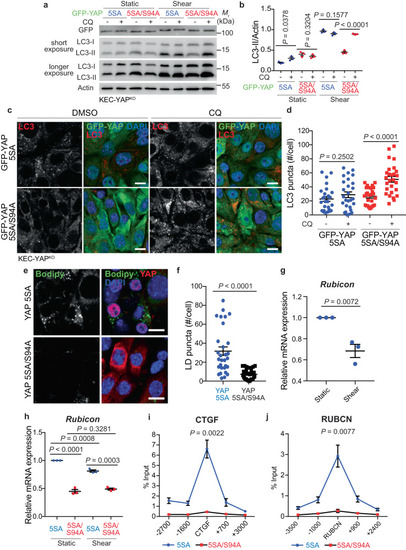
The expression of a constitutively active form of YAP inhibits autophagy flux during shear stress. |
|
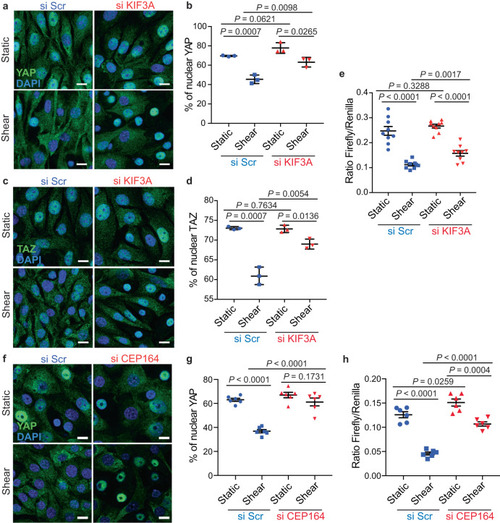
YAP/TAZ inactivation by shear stress requires a functional primary cilium. |
|
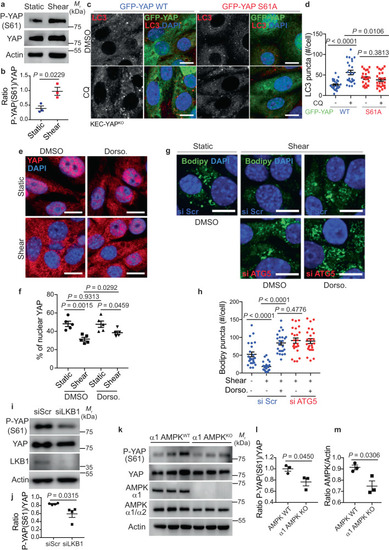
AMPK-dependent phosphorylation of YAP at S61 regulates autophagy upon fluid flow. |
|
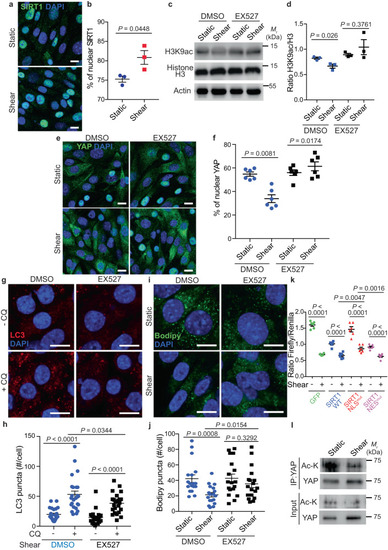
SIRT1 induces YAP nuclear exclusion upon fluid flow. |
|
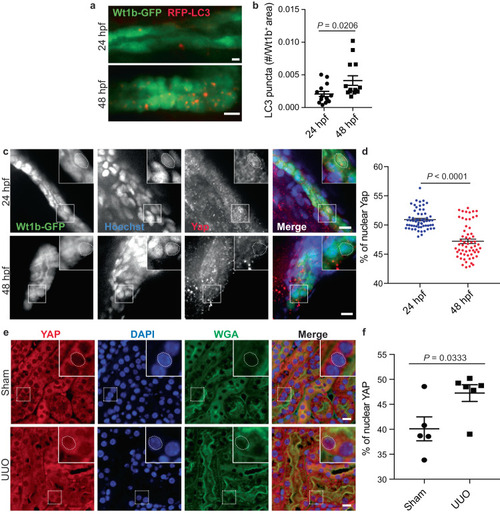
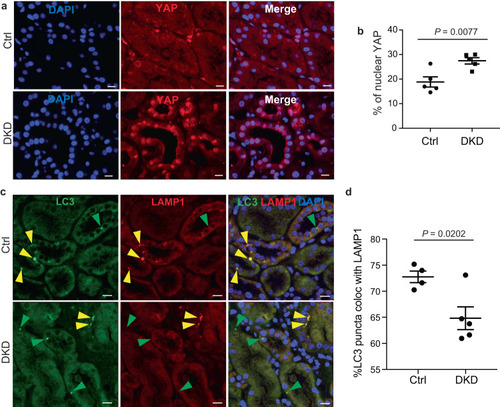
YAP subcellular localization is associated to autophagy activity in vivo. |
|
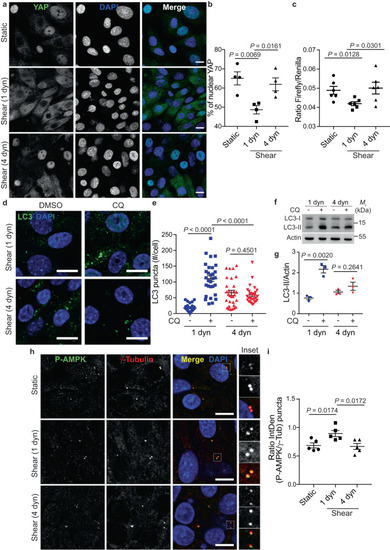
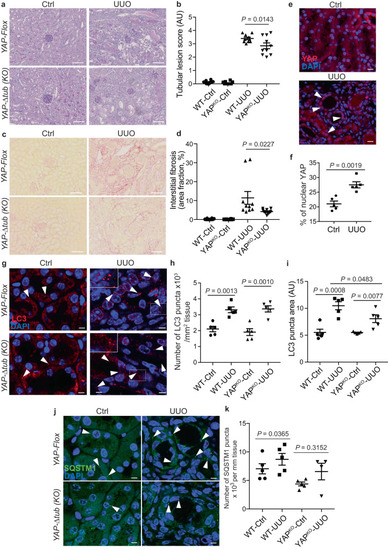
Pathological flow induces YAP nuclear translocation and inhibits autophagy. |
|
YAP reactivation upon chronic kidney disease is correlated with autophagy inhibition. |
|
The YAP/autophagy crosstalk promotes renal interstitial fibrosis during unilateral ureteral obstruction. |