- Title
-
The loss of DHX15 impairs endothelial energy metabolism, lymphatic drainage and tumor metastasis in mice
- Authors
- Ribera, J., Portolés, I., Córdoba-Jover, B., Rodríguez-Vita, J., Casals, G., González-de la Presa, B., Graupera, M., Solsona-Vilarrasa, E., Garcia-Ruiz, C., Fernández-Checa, J.C., Soria, G., Tudela, R., Esteve-Codina, A., Espadas, G., Sabidó, E., Jiménez, W., C Sessa, W., Morales-Ruiz, M.
- Source
- Full text @ Commun Biol
|
|
|
|
|
|
|
|
|
|
|
|
|
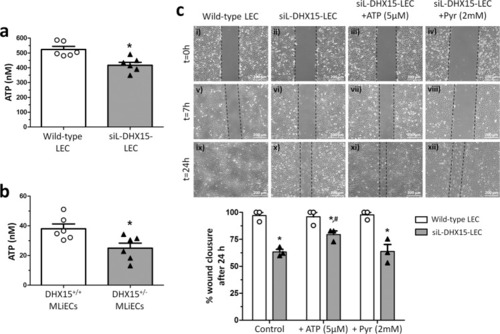
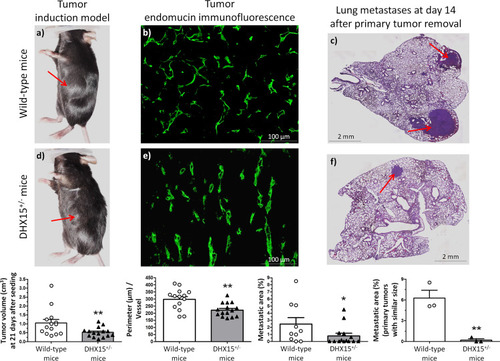
Macroscopic images of tumor size in wild-type ( |

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. EXPRESSION / LABELING:
|