- Title
-
Preconditioning boosts regenerative programmes in the adult zebrafish heart
- Authors
- de Preux Charles, A.S., Bise, T., Baier, F., Sallin, P., Jaźwińska, A.
- Source
- Full text @ Open Biol.
|
Thoracotomy triggers cell proliferation in the intact zebrafish heart. (a,b) Representative sections of hearts of transgenic fish cmlc2:DsRed2-nuc (red), which demarcate cardiomyocyte (CM) nuclei, labelled with the G1/S-phase marker MCM5 (green). (c,d) Representative sections of hearts after one week of BrdU (green) treatment. Mef2 staining (red) was performed to differentiate CM nuclei from non-CM nuclei. (a′,b′,c′,d′,a′′,b′′,c′′,d′′) Higher magnifications of the framed area shown in the images that are labelled with the same letter without the prime symbol. The same rule applies to all the subsequent figures. Arrows indicate double-positive nuclei. (e-h) Quantification of MCM5- and BrdU-positive CM and non-CM nuclei. (n ≥ 4 hearts; ≥2 sections per heart; ***p < 0.001). |
|
Thoracotomy stimulates mitotic events in the intact heart without apoptotic turnover of the newly generated cells. (a) Representative image of a mitotic CM in an intact heart of transgenic fish cmlc2:EGFP at 7 dpt. Orthogonal projections demonstrate a colocalization between PH3 (red), GFP (green) and DAPI (blue) staining. (b,c) Quantification of PH3-positive CM and non-CM nuclei at 7 dpt. Mef2 was used as a CM nuclei marker. (d-f) Representative images of the TUNEL assay (green) at 4 dpci (days post-cryoinjury; positive control for apoptosis with the post-infarcted area surrounded with a dashed line), in uninjured hearts and at 4 dpt. (g) Quantification of TUNEL-positive nuclei in hearts at 4 dpci, in uninjured hearts and at different time points post-thoracotomy (n ≥ 4 hearts; ≥2 sections per heart; n.s., non-significant; ***p < 0.001). EXPRESSION / LABELING:
|
|
Thoracotomy triggers the remodelling of the heart architecture. (a,b) Uninjured and 30 dpt heart sections of transgenic zebrafish cmlc2:DsRed2-nuc (red) after one month of BrdU treatment (green). (c,d) Representative sections of hearts labelled with Phalloidin (red), a muscle marker, at 30 dpt. (e) Representative section of an uninjured heart labelled with haematoxylin/eosin (h,e) staining. Compact myocardium (Comp. MC) is composed of a dense layer of CMs, surrounding the trabecular myocardium (Trab. MC) with a spongy lumen. (a-e) Dashed lines separate compact and trabecular myocardium. (f,g) Percentage of BrdU-positive CMs in whole myocardium (f) or in the two distinct myocardium compartments (g). (h-j) Morphometric measurements of hearts at 30 dpt. No difference in heart size was noticed (h), but a thickening of the compact myocardium was observed (i). (j) The density of CMs (number of CMs per 10 000 µm2 of cardiac muscle) is higher at 30 dpt in comparison to uninjured control (n e 4 hearts; e2 sections per heart; *p < 0.05; ***p < 0.001). |
|
A single intraperitoneal injection of immunogenic particles triggers the reentry of CMs into the cell cycle. (a-d) Representative heart sections of uninjected transgenic cmlc2:DsRed2-nuc (red) fish controls (a) or injected with Hank′s solution (b), LPS (c) or Zymosan (d) labelled with the cell-cycle marker MCM5 (green). Dashed lines separate compact and trabecular myocardium. Arrows indicate double-positive nuclei. (e,f) Quantification of MCM5-positive CM and non-CM nuclei. (g) Sublocalization of MCM5-positive CMs within the distinct myocardium compartment, the compact (Comp. MC) and trabecular (Trab. MC) myocardium (n ≥ 4 hearts; ≥2 sections per heart; n.s., non-significant; *p < 0.05; ***p < 0.001). |
|
A single intraperitoneal injection of immunogenic particles induces a remodelling of the heart architecture. (a-c) Representative sections of hearts labelled with Phalloidin, a muscle marker, at 30 dpi. Dashed lines separate compact and trabecular myocardium. (d-f) Morphometric measurements of hearts at 30 dpi. No difference in heart size was noted (d), but a thickening of the compact myocardium was observed in fish injected with either LPS or Zymosan. (f) The density of CMs (number of CMs per 10 000 µm2 of cardiac muscle) was higher at 30 dpi in the compact myocardium of LPS- or Zymosan-injected fish (n ≥ 4 hearts; ≥2 sections per heart; n.s., non-significant; *p < 0.05; ***p < 0.001). PHENOTYPE:
|
|
The expression of cardioprotective genes is induced in the epicardium after thoracotomy. (a-d) Representative images of in situ hybridization on heart sections with antisense txn and cxcl12a probes. (e,f) The relative expression level of hmox1a and hpsa5 was tested by qRT-PCR (n ≥ 3; each replicate is a pool of six ventricles; **p < 0.01). EXPRESSION / LABELING:
|
|
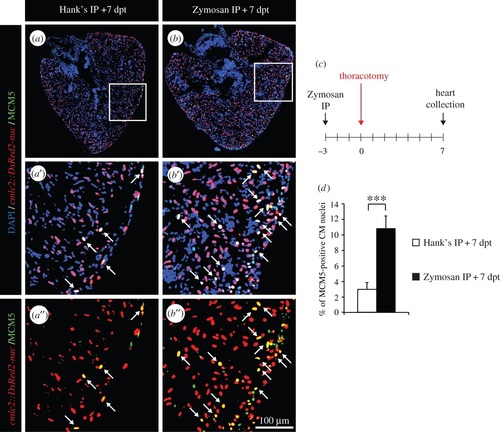
The effects on two subsequent preconditioning stimuli are additive. (a,b) Representative heart sections of transgenic cmlc2:DsRed2-nuc (red) fish labelled with the G1/S-phase marker MCM5 (green) at 7 dpt. Arrows indicate double-positive nuclei. (c) Experimental design. Fish were injected intraperitoneally (IP) with Zymosan 3 days before the thoracotomy procedure and cell-cycle activity was tested at 7 dpt. (d) Quantification of MCM5-positive CM nuclei at 7 dpt (n ≥ 4 hearts; ≥ 2 sections per heart; ***p < 0.001). EXPRESSION / LABELING:
|
|
Heart regeneration is improved in fish subjected to thoracotomy a few days before the cryoinjury. (a,b) Representative sections of hearts of transgenic cmlc2:DsRed2-nuc (red) fish labelled with the G1/S-phase marker MCM5 (green) at 7 dpci. Arrows indicate double-positive nuclei. (c,d) Experimental design. (c) Fish were subjected to thoracotomy at 7 or 2 days before the cryoinjury procedure. (d) Fish were injected intraperitoneally (IP) with Zymosan at 3 days before the cryoinjury procedure. (e,f) Representative sections of hearts labelled with the muscle marker Phalloidin (red) and embCMHC (green), a marker of undifferentiated CMs. (g,i) Scatter plot representing the percentage of MCM5-positive CM nuclei as a function of the cryoinjured area while using thoracotomy (g) (grey squares = 7 dpci; red squares = 2 dpt + 7 dpci; black squares = 7 dpt + 7 dpci) or Zymosan-IP (i) (grey squares = Hank′s + 7 dpci; black squares = Zymosan + 7 dpci) as preconditioning stimuli. (h,j) Quantification of the embCMHC-positive area after thoracotomy (h) or Zymosan-IP injection (j). A dashed line encircles the cryoinjured area (n ≥ 4 hearts; ≥2 sections per heart; **p < 0.01). EXPRESSION / LABELING:
|
|
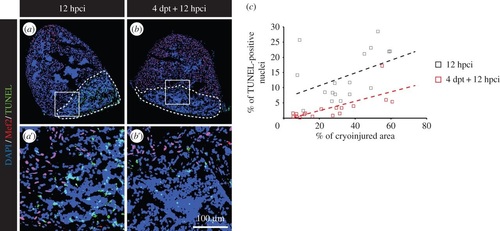
Preconditioning enhances cell survival after cryoinjury. (a,b) Representative pictures of the TUNEL assay (green) at 12 hpci in uninjured (a) or preconditioned fish (b). Mef2 labelling (red nuclei) is absent from the cryoinjured area. A dashed line encircles the wound. (c) Scatter plot representing the % of TUNEL-positive nuclei as a function of the cryoinjured area while using thoracotomy (black squares = 12 hpci; red squares = 4 dpt + 12 hpci) as preconditioning stimulus. EXPRESSION / LABELING:
|
|
Thoracic wound closes spontaneously within 3-4 days after thoracotomy. Photographs of the ventral side of the same fish at different time points after performing 1 mm-long incision through the body wall. (a) At 6 hours post thoracotomy (hpt), the thorax has an open wound, seen as a dark window through the whitish dermis. A transparent epithelium already starts to cover the wound, reducing the diameter of the hole (encircled area). (b) At 3 days post thoracotomy (dpt), the wound has been completely sealed by the sheet of epidermis. (c) At 5 dpt, dermis has contracted around the wound. (d) At 7 dpt, the thoracic wound has been almost perfectly healed by the restoration of the dermal tissue. |