- Title
-
Long-Chain Acyl-CoA Synthetase 4A Regulates Smad Activity and Dorsoventral Patterning in the Zebrafish Embryo
- Authors
- Miyares, R.L., Stein, C., Renisch, B., Anderson, J.L., Hammerschmidt, M., and Farber, S.A.
- Source
- Full text @ Dev. Cell
|
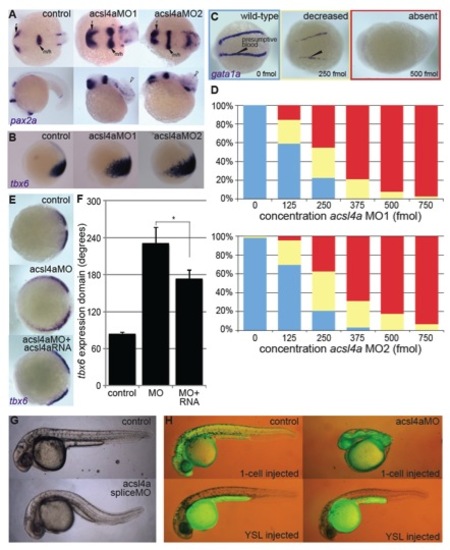
Acsl4a Is Necessary for Proper Dorsoventral Patterning (A) acsl4a expression throughout early embryonic development. Whole-mount mRNA in situ hybridizations. Expression pattern was consistent over multiple experiments (n = 7) and between two separate riboprobes targeting acsl4a. Lateral views, animal pole to the top. e, epiboly. (B) Morphological defects in dorsoventral patterning caused by perturbation of acsl4a expression. Phenotypic classes were assigned as described elsewhere ( Kishimoto et al., 1997). Representative bright-field images of wild-type embryos (24 hpf) that are dorsalized (class C4 phenotype shown) when injected with acsl4a MO and ventralized (class V3 phenotype shown) when injected with acsl4a mRNA. Embryos injected with a catalytically inactive version of acsl4a (acsl4aCI) mRNA and control siblings develop normally. Arrowhead indicates wound-up trunk and arrow indicates expanded blood island. 500 fmol acsl4a MO; n = 8 experiments (406 embryos); 79%C5, 8%C4, 11%C3, 1%C2, and 1%C1; 1.2–1.5 ng acsl4a mRNA; n = 3 (101 embryos); 6%V4, 23%V3, 25%V2, 45%V1, 2%N; 1.2–1.5 ng acsl4aCI mRNA; n = 3 (123 embryos); 100% N. (C) Fate map of the gastrula-stage zebrafish embryo (Kimmel et al., 1990 Schier and Talbot, 2005). Blue represents ventral fates and red represents dorsal fates. (D–G) Whole-mount mRNA in situ hybridization of markers of dorsal and ventral tissues in 80% epiboly stage embryos, either control or acsl4a MO-injected. Animal pole view, dorsal to the right. (D) tbx6, a marker of dorsal presumptive paraxial mesoderm, is expanded ventrally in acsl4a MO-injected embryos. 95% of acsl4a MO-injected (750 fmol) embryos displayed expansion >180 degrees (n = 8; 323 embryos). (E) eve1, a marker of ventral mesoderm, is reduced in acsl4a MO-injected (500 fmol) embryos (97%; n = 6; 272 embryos). (F) foxb1.2, a marker of neural plate/dorsal ectoderm, is expanded ventrally in acsl4a MO-injected (500 fmol) embryos (93%; n = 4; 135 embryos). (G) gata2a, a marker for epidermis/ventral ectoderm, is reduced in acsl4a MO-injected (500 fmol) embryos (98%; n = 4; 181 embryos). (H) pax2a and myod1 expression in four-somite stage embryos. Dorsal view, anterior to the left. pax2a marks m/h, midbrain/hindbrain boundary; o, otic placode; and p, pronephros. myod1 marks a, adaxial cells. 98% of acsl4a MO-injected (500 fmol) embryos had expansion of pax2a marking m/h (n = 7; 125 embryos). Seventy-five percent of acsl4a MO-injected (500 fmol) embryos had loss and 25% had reduced expression of pax2a marking pronephros (n = 5; 86 embryos). (I) myod1 expression in 15-somite stage embryos. Dorsal view, anterior to the left. s, somites. Ninety-six percent of acsl4a MO-injected (500 fmol) embryos had expanded somites (n = 10; 138 embryos). See also Figures S1 and S2. EXPRESSION / LABELING:
PHENOTYPE:
|
|
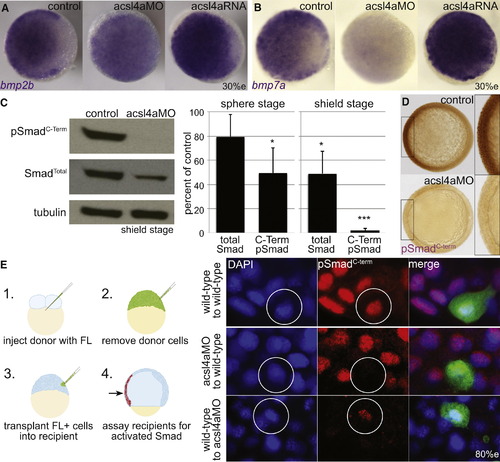
Bmp Expression and Signaling Is Dependent on Acsl4a (A and B) Whole-mount mRNA in situ hybridization of bmp transcripts in control embryos and embryos injected with acls4a MO (500 fmol) or acsl4a mRNA (1.25 ng). In acls4a morphants, the ventral-to-dorsal gradient of (A) bmp2b and (B) bmp7a is attenuated (in 87% and 78%, respectively) while acls4a overexpression enhances bmp expression (in 77% and 75%, respectively). n = 4; 88–119 embryos. Animal pole view, dorsal to the right (30% epiboly). (C) Western blot of C-terminally phosphorylated Smad1/5/8 (pSmadC-Term) and total Smad1/5/8 (SmadTotal) from shield-stage control embryos and embryos injected with 750 fmol acsl4a MO (left). Quantification of three (sphere) or five (shield) experiments (right). Data are represented as mean ± SE. p < 0.05 p < 0.001; one sample t test with Bonferroni correction (sphere stage MO = 500 fmol, shield stage MO = 500–750 fmol). (D) Whole-mount immunostain against pSmadC-Term at 80% epiboly. Staining is reduced in morphants (500 fmol) when compared to control siblings (98%; n = 5; 155 embryos). Animal pole view, dorsal to the right (80% epiboly). Inset is a 2× magnification of the ventral-most region. (E) Acsl4a is needed in the Bmp ligand-receiving cell for Smad activation. (Left) Schematic of transplantation experiment. (Right) pSmadC-Term immunofluorescence in transplanted cells in ventral equatorial regions of host embryos (80% epiboly). Top panel: wild-type cells transplanted into wild-type embryos have normal pSmadC-Term staining (26/26 embryos, three independent experiments). Middle panel: Morphant cells transplanted into wild-type embryos lack pSmadC-Term staining, whereas adjacent cells have normal pSmadC-Term staining (16/16 embryos, n = 3). Bottom panel: Wild-type cells transplanted into morphants have pSmadC-Term staining, whereas adjacent morphant cells lack pSmadC-Term signals (17/17 embryos, n = 3). Wild-type cells transplanted into morphants show reduced pSmadC-Term staining compared to wild-type cells transplanted into wild-type hosts (compare bottom and top panels). FL, fluorescein dextran. See also Figure S3. |
|
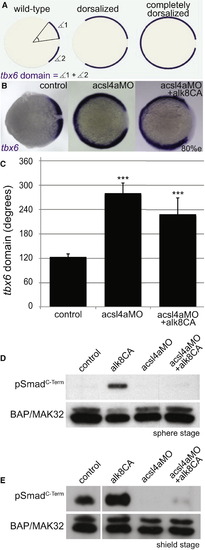
Acsl4a Acts Downstream of Bmp Receptor Activation (A) The extent of dorsalization is measured by adding the angles of the arcs of dorsal marker tbx6 expression on either side of the tbx6 negative midline at 80% epiboly. Embryos are considered completely dorsalized if the arcs meet on the ventral side. (B and C) Constitutively active Bmp receptor does not rescue the phenotype caused by acsl4a depletion. (B) tbx6 expression in a control embryo, embryo injected with acsl4a MO alone (750 fmol), or acsl4a MO combined with constitutively active alk8 (alk8CA, 1.5 ng) mRNA. Vegetal pole view, dorsal to the right (80% epiboly). (C) Quantification of tbx6 expression domain. Data are represented as mean of experimental means ± pooled SE (n = 3–8; 12–80 embryos/experiment). ANOVA with Dunnett post hoc test; p < 0.0001, compared to control. (D and E) Western blots (n = 3) of C-terminally phosphorylated Smad1/5/8 (pSmadC-Term) from embryos injected with alk8CA mRNA (20-40 pg), acsl4a MO (500 fmol), or acsl4a MO and alk8CA mRNA combined. Anti-BAP/MAK32 is the loading control. (D) Lysates from sphere-stage embryos. (E) Lysates from shield-stage embryos. An extraneous lane between control and alk8CA is omitted. See also Figure S4. |
|
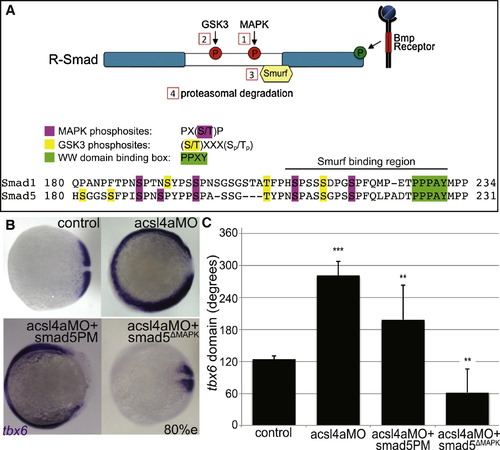
Acsl4a Acts via R-Smad Phosphorylation at MAPK Consensus Sites (A) Model of R-Smad regulation by phosphorylation. (Top) The Bmp receptor positively regulates (green) R-Smad activity by phosphorylating the C terminus. Negative regulation (red) of R-Smad occurs in sequential steps: (1) MAPK phosphorylates the R-Smad linker. (2) GSK3 phosphorylates the linker upstream of MAPK phosphoresidues. (3) Smurf1/2 E3 ubiquitin ligase recognizes R-Smad after phosphorylation by MAPK and/or GSK3. (4) Smurf1/2 polyubiquitinates R-Smad, leading to its proteasomal degradation. (Bottom) Sequence alignment of zebrafish Smad1 and Smad5 linker regions with important residues highlighted. (B) tbx6 expression in a control embryo, embryo injected with acsl4a MO alone (750 fmol), acsl4a MO combined with a C-terminal phosphomimic smad5 mRNA (smad5PM; 1.5 ng), and acsl4a MO combined with a MAPK-insensitive version of smad5 mRNA (smad5ΔMAPK; 1.5 ng). Vegetal pole view, dorsal to the right (80% epiboly). (C) Quantification of tbx6 expression domain. Data are represented as mean of experimental means ± pooled SE (n = 3–8, 12–80 embryos/experiment). ANOVA with Dunnett post hoc test; p < 0.01, p d 0.005, p < 0.0001; compared to control. See also Figure S5. |
|
Acsl4a Regulates p38 MAPK Activity and R-Smad Linker Phosphorylation (A) An acsl4a MO-dependent increase in MAPK activity would result in phosphorylation of R-Smad and recruitment of Smurf ubiquitin ligase. An increase in MAPK activity can be assayed by antibodies specific to activated MAPK. (B) p38 MAPK activity is upregulated in acsl4a morphants (750 fmol) at high stage. Western blot for phosphorylated (Thr180/Tyr182) p38 MAPK (active-p38 MAPK; n = 6). (C) Smad5ΔSmurf lacks the WW domain-binding box (PPPAY→ΔΔΔΔΔ) recognized by Smurf E3 ubiquitin ligase, thus it is prevented from linker-mediated degradation. An increase in MAPK phosphorylation of R-Smad can be assayed with a phosphospecific antibody, pSmadMAPK. (D) Smad5ΔSmurf is phosphorylated at the MAPK consensus site (Ser215) after acsl4a knockdown. Western blot for MAPK phosphorylated Smad (pSmadMAPK; gift of de Robertis) at sphere stage. acsl4a MO injection (750 fmol) results in increased pSmadMAPK staining. Alpha tubulin is the loading control. See also Figure S6. EXPRESSION / LABELING:
|
|
Acsl4a Regulates GSK3 Activity (A) An acsl4a MO-dependent increase in GSK3 activity would result in phosphorylation of R-Smad and recruitment of Smurf ubiquitin ligase. There are inhibitory phosphorylation sites (GSK3α Ser21/GSK3β Ser9) that control GSK3 activity level, thus an antibody that recognizes the inhibitory phosphorylation (inactive GSK3) can assay GSK3 activity. (B) GSK3 activity is increased in acsl4a morphants (500 fmol). (Left) Western blot for inhibitory phosphorylation (GSK3αSer21/GSK3β Ser9) of GSK3 (inactive GSK3). (Right) Western blot for total GSK3β. BAP/MAK32 is the loading control. (C) GSK3 is inhibited by Akt. An acsl4a MO-dependent decrease in Akt activity would result in decreased inhibition (thus activation) of GSK3. A decrease in Akt activity can be assayed by an antibody specific to active (phosphorylated on Ser473) Akt. (D) Akt activity is decreased in acsl4a morphants (500 fmol). Western blot for phosphorylated (Ser473) Akt (active AKT). BAP/MAK32 is the loading control. (E) Quantification of blots from (B) and (D). Data are mean ± SE of three to four experiments and represented as percent of control (p < 0.05 p < 0.001; one sample t test with Bonferroni correction). (F–H) Constitutively active, myristoylated Akt rescues the acsl4a MO’s dorsalized phenotype. (F) Constitutively active Akt (50–100 pg) decreases GSK3 activity (increase in inactive GSK3) in acsl4a MO-injected (333 fmol) embryos. Data are represented as mean ± SD (n = 2). (G) Expression of tbx6 in a control embryo, embryo injected with acsl4a MO alone (333 fmol), embryo injected with constitutively active akt mRNA (50–100 pg; aktCA), and acsl4a MO with aktCA. Vegetal pole view, dorsal to the right (80% epiboly). (H) Quantification of tbx6 expression domain. Data are represented as mean of experimental means ± pooled SE (n = 3; 20–24 embryos/experiment). ANOVA with Dunnett; p < 0.05, p < 0.0001. EXPRESSION / LABELING:
|
|
Identification and embryonic expression of zebrafish acsl4a, which is necessary for proper dorsoventral patterning, related to Figure 1. EXPRESSION / LABELING:
|
|
acsl4a MOs specifically inhibit maternal acsl4a transcripts essential for dorsoventral patterning defect, related to Figure 1. PHENOTYPE:
|
|
Bmp signaling is dependent on Acsl4a, related to Figure 2. Representative western blots of C-Terminal phosphorylated (pSmadC-Term) and pan Smad1/5/8 (SmadTotal) from control embryos and embryos injected with alk8CA mRNA (20-40 pg), acsl4a MO (500 fmol). Anti-BAP/MAK32 is shown as a loading control. (A) Lysates from sphere-stage embryos. (B) Lysates from shield-stage embryos. |
|
Acsl4a is epistatic to the Bmp receptor Alk8, related to Figure 3. PHENOTYPE:
|
|
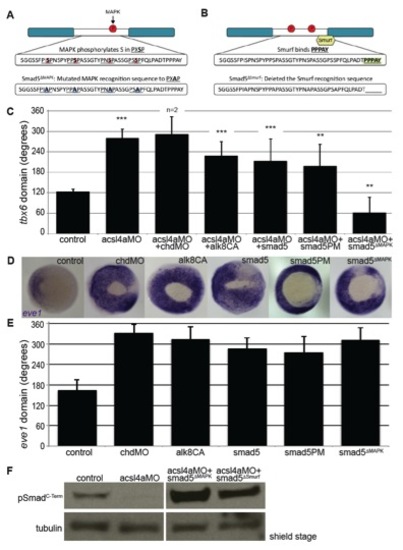
Epistasis analysis of Bmp signaling cascade, related to Figure 4. (A-B) Diagrams of Smad5 constructs, showing mutated residues in the linker region. (A) MAPK-insensitive Smad5: Smad5ΔMAPK (B) Non-degradable Smad5: Smad5ΔSmurf (C) Quantification of the angle of tbx6 expression from whole-mount in situ hybridization after injection with acsl4a MO (750 fmol) alone or combined with chordin morpholino (chdMO 20fmol), constitutively active alk8 mRNA (alk8CA 1.5 ng) mRNA, C-terminal phosphomimic smad5 (smad5PM 1.5 ng) mRNA, and MAPK-insensitive smad5 (smad5ΔMAPK 1.5 ng) mRNA. Data are represented as mean of experimental means ± pooled SE. For acsl4a MO + chordin MO, data are represented as mean of experimental means ± pooled SD (n=2-8 experiments, 12-80 embryos/experiment). ANOVA with Dunnett post-hoc test was performed; acsl4a MO + chordin MO data was excluded from analysis. ** p d0.005, *** p<0.0001: compared to control. (D-E) Constructs used for rescue analysis in Figures 2B&C, 4B&C and S5A ventralize embryos to a similar extent. (D) Whole-mount in situ hybridization of ventral mesoderm marker eve1 after injection with chordin MO or mRNAs from Figures 2B&C, 4B&C and S5A. Injections were performed in parallel (i.e., with the same injection needle) with those in Figures 2B&C, 4B&C and S5A. Vegetal pole view, dorsal to the right (80% epiboly). (E) Data are represented as mean of experimental means ± pooled SD (n=2-7 experiments, 8-47 embryos/experiment). (F) Smad5 mutant constructs are phosphorylated by the Bmp receptor independent of Acsl4a. Representative western blot (n=4) of C-Terminal phosphorylated Smad (pSmadC-Term) from control embryos, embryos injected with acsl4a MO (750 fmol) or embryo injected with acsl4a MO combined with smad5ΔMAPK (1.5 ng) or smad5ΔSmurf (1.5 ng) mRNA. Anti-alpha tubulin is shown as a loading control. An extraneous lane between acsl4a MO and acsl4a MO + smad5ΔMAPK is omitted. |
|
Acsl4a does not activate Erk1/2 or Jnk MAPK cascades, related to Figure 5. (A) Erk1/2 is not activated in acsl4a morphants. Representative western blot of phosphorylated Erk1/2 from control embryos and embryos injected with acsl4a MO (500 fmol) at sphere stage. Anti-BAP/MAK32 is shown as a loading control. An extraneous lane is omitted. pERK1/2 levels in acsl4a MO-injected embryos are 90±19% of control (not significant, n=3). (B) Jnk signaling cascade is not activated in acsl4a morphants. Representative western blot of phosphorylated MKK4 from control embryos and embryos injected with acsl4a MO (750 fmol) at sphere stage. Blots were stripped and probed with alpha-tubulin as a loading control. pMKK4 levels in acsl4a MO-injected embryos are 1.57 ± .82% of control (not significant, n=4). (C) Representative western blot (n=3) testing the antibody against MAPK phosphorylated hSmad1 (pSmad”MAPK) (gift of Eddie de Robertis (Fuentealba et al., 2007)) on zebrafish Smad constructs (1 ng). An extraneous lane between smad1”Smurf and smad1”Smurf”MAPK is omitted. EXPRESSION / LABELING:
|
|
Acsl4a does not significantly alter Smad2/3 signaling, related to Figure 7. (A) Whole-mount mRNA in situ hybridization of ntl in 50% epiboly stage embryos, uninjected (control) or injected with 500 fmol acsl4a MO. Animal pole view. 96% of acsl4a MO-injected embryos appear wild-type (n=3 experiments; 127 embryos). (B) Sequence alignment of zebrafish Smad1/5 and Smad2/3 linker regions with important residues highlighted. EXPRESSION / LABELING:
|
Reprinted from Developmental Cell, 27(6), Miyares, R.L., Stein, C., Renisch, B., Anderson, J.L., Hammerschmidt, M., and Farber, S.A., Long-Chain Acyl-CoA Synthetase 4A Regulates Smad Activity and Dorsoventral Patterning in the Zebrafish Embryo, 635-647, Copyright (2013) with permission from Elsevier. Full text @ Dev. Cell