- Title
-
Ryanodine receptors, a family of intracellular calcium ion channels, are expressed throughout early vertebrate development
- Authors
- Wu, H.H., Brennan, C., and Ashworth, R.
- Source
- Full text @ BMC Res. Notes
|
Zebrafish ryr mRNA can be detected from very early developmental stages through to adulthood. (a) Temporal expression pattern of zebrafish ryrs using end-point PCR analysis and isoform specific primers. Total RNA was isolated from different developmental stages, as indicated, and reverse transcribed. 1.5 μg of cDNA was synthesised and isoform specific primers for ryr1a, ryr1b, ryr2a, ryr2b and ryr3 used. Numbers in the right panel indicate the molecular mass of PCR products. β-actin was used as an internal control. The figure shows a representative result of replicates from at least three experiments. (b) Expression of zebrafish ryr2a and ryr2b in the brain (BR) and body (BO) of a 3 months old adult zebrafish. β-actin was used as an internal control. Numbers in the right panel indicate the molecular mass of RT-PCR products. (c) Maternal expression of ryr3 mRNA was observed as staining (arrows) within the dividing cells of 2-2.25 hpf embryos (64 and 128-cell stage). The specificity of the signal is compared to the ryr3 sense probes. |
|
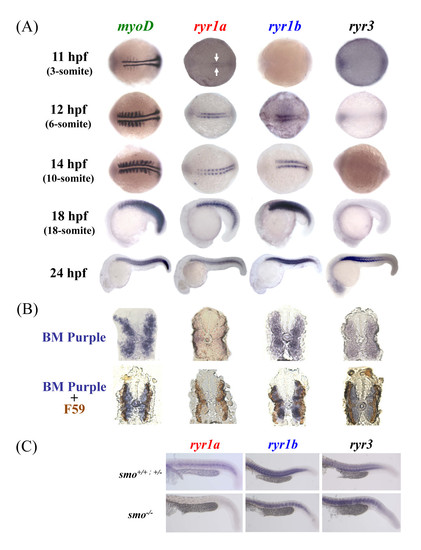
Zebrafish ryr1a, ryr1b and ryr3 mRNA is localised to embryonic skeletal muscle. (a) Expression of zebrafish myoD, ryr1a, ryr1b and ryr3 was examined using whole mount in situ hybridisation. Expression of ryr1a mRNA is detectable in the adaxial cells of 11 hpf embryos (3-somite, arrows), whereas ryr1b mRNA expression is present in cells adjacent to the notochord from 12 hpf (6-somite stage) embryos. Expression of ryr3 mRNA was only evident at 24 hpf, with the strongest staining observed in the anterior somites. The embryos at 11 and 12 hpf are orientated so that the anterior is to the left. (b) Cross-sections showing myoD, ryr1a, ryr1b and ryr3 in-situ mRNA hybridisation (above) and double immunostained with the F59 antibody/HRP labeling (below) in 24 hpf embryos. (c) ryr1a, ryr1b and ryr3 mRNA expression in wildtype (smo+/+), heterozygote (smo+/-) and homozygous (smo-/-) mutants at 24 hpf. There is no ryr1a mRNA expression in the smo-/- mutant, compared to wildtype and heterozygous embryos at 24 hpf. EXPRESSION / LABELING:
|
|
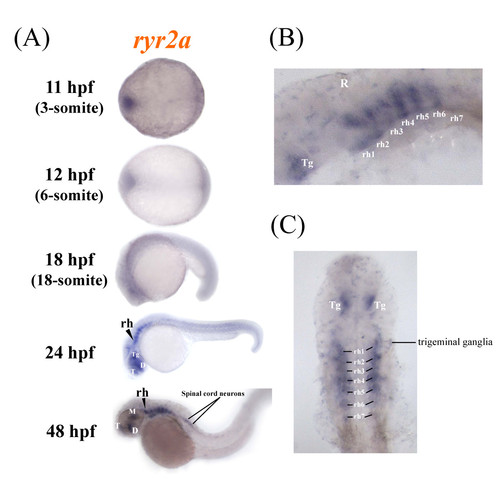
Zebrafish ryr2a is expressed in the central nervous system. The spatial distribution of ryr2a mRNA in the zebrafish embryo was examined using whole mount in situ hybridisation. (a) Specific staining was observed initially in localised regions of the brain, specifically the telencephalon (T), diencephalon (D), mesencephalon (M), tegmentum (Tg) and rhomobomeres (rh) of whole mount embryos at 24 and 48 hpf. (b) Lateral and (c) dorsal views (eyes removed) of the brain at 48 hpf revealed that ryr2a expression was present in each of the seven rhombomere segments, the tegmentum, trigeminal ganglia and anterior portion of the spinal cord. Embryos are orientated so that anterior is to the left. EXPRESSION / LABELING:
|
|
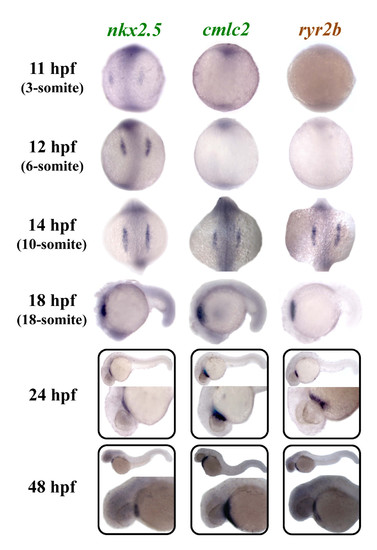
Zebrafish ryr2b is expressed in the differentiating cardiac muscle. Expression of zebrafish nkx2.5, cmlc2, and ryr2b was examined using whole mount in situ hybridisation. Expression of nkx2.5 was observed in myocardial precursors from 11 hpf (3-somite stage) onwards, whereas cmlc2 and ryr2b were expressed in differentiating cardiac tissue from 14 hpf (10-somite stage) onwards. 11, 12 and 14 hpf embryos are orientated so that the anterior is to the top, whereas in 18, 24 and 48 hpf embryos anterior is to the left. EXPRESSION / LABELING:
|
|
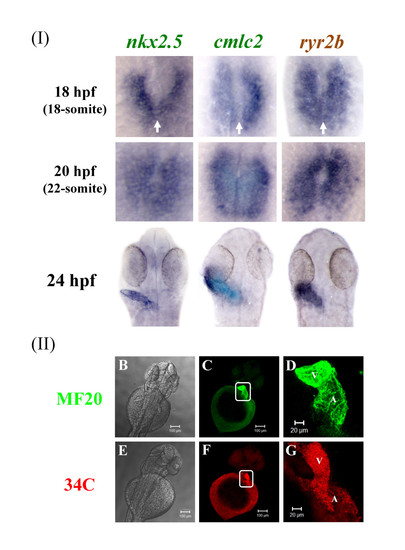
Zebrafish ryr2b mRNA is expressed during early heart formation and the RyR protein throughout the heart at later stages. (a) Expression of nkx2.5, cmlc2, and ryr2b in 18 hpf (18-somite stage) was observed in the bilateral cardiac primordial cells. At this stage the bilateral cardiac primordial cells make contact and begin to fuse (arrows). The posterior portions fuse initially followed by anterior portions to create a central lumen and cardiac cone by 20 hpf (22-somite stage). A linear heart tube has formed by 24 hpf and ryr2b and cmlc2+ cells are expressed throughout the heart at this stage. 18- and 22-somite stage embryos, in which the tail was removed but the yolk sac left intact, were orientated so that anterior is to the top. Dorsal view of flat mounted embryos at 24 hpf. (b&e) Brightfield images of the anterior portion of the zebrafish embryo with the yolk sac still intact and dorsal side uppermost. Embryos were fixed and stained at 48 hpf to reveal either myosin (c-d) or RyRs (f-g), the images within the white boxes are shown in greater detail (d & g). By 48 hpf RyRs are expressed throughout the two chambers, atrium (A) and ventricle (V) of the heart. Images were taken using X10 (b,c & e,f) and X20 (d & g) objectives. EXPRESSION / LABELING:
|
|
ryr1a, ryr1b and ryr3 mRNA is differentially expressed in the developing skeletal muscle. Double labelling in wholemount embryos at 24 hpf was performed using probes to myhz1, ryr1a, ryr1b and ryr3 and Fast Red as a substrate (red) followed by immunostaining using the F59 antibody and a fluorescent secondary (green). Images show Z-stacks of whole-mount double-labelled embryos (top row) or sections (bottom row) from dissected embryos. Cross-sectional images revealed that ryr1a co-localised exclusively with slow muscle staining, whereas myhz1, a marker of fast muscle, was not expressed in the slow muscle. Furthermore ryr1b expression could be observed throughout both muscle types whereas ryr3 did not co-localise with the slow muscle staining and appeared to be expressed exclusively in the fast muscle. Scale bars = 20 μm, unless otherwise indicated. |
|
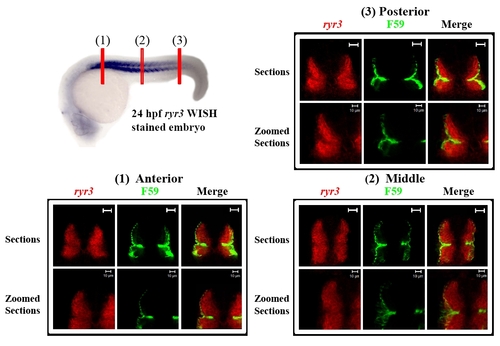
ryr3 mRNA expression is confined to the fast muscle fibres throughout the myotome at 24 hpf. Double fluorescent cross-sections (1-3) were prepared by labelling a 24 hpf zebrafish embryo with a fluorescence substrate (i.e. FastRed; red) for ryr3 in situ hybridisation and the F59 antibody (green) for immunostaining. The position of cross-sections 1-3 are illustrated in the 24 hpf ryr3 WISH labelled embryo (top left) which has been stained with BM purple and laterally orientated with anterior to the left. Scale bars = 20 μm, unless otherwise indicated. |