- Title
-
Semaphorin3D regulates invasion of cardiac neural crest cells into the primary heart field
- Authors
- Sato, M., Tsai, H.J., and Yost, H.J.
- Source
- Full text @ Dev. Biol.
|
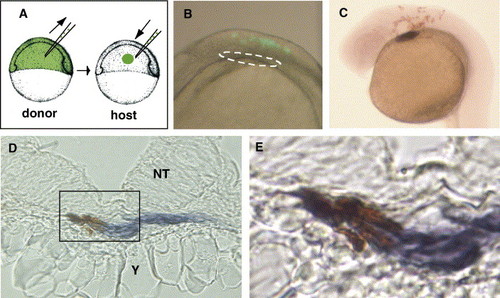
Neural crest cells migrate to the primary heart field and express cmlc2. (A) Transplantation strategy. At the shield stage, 10–50 prospective neural crest cells were transplanted from fluorescein dextran donors into the orthotopic region of hosts. (B) 8 SS embryo, DIC and epi-fluorescent (lateral view, anterior at left, dorsal at top). Lineage-labeled cells (green) were in neural crest and neural tube at hindbrain level, but not in anterior LPM (white circle). (C) 26 SS embryo, lineage-labeled cells (anti-fluorescein antibody, brown) and cardiomyocytes (cmlc2, purple) were detected. (D) Transverse section through the primary heart field of 26 SS embryo with lineage-labeled neural crest cells (brown) expressing cmlc2 (purple). NT = neural tube, Y = yolk. (E) Magnified image of box in panel D. EXPRESSION / LABELING:
|
|
sema3D-aug morphants have cardiac neural crest defects. (A, A′) At 8 SS, sema3D RNA is expressed in rhombomere 3–5 and in neural crest (black arrow). Anterior left, dorsal up. (B–F′) Phenotypic analyses of wild-type (B–F) and sema3D-augMO morphants (B′–F′). Morphants had reduced crestin expression in cranial crest (8SS, arrowheads, B and B′), pericardiac edema (4 dpf, arrow c′) and shortened neck indicative of pharyngeal arch defects, decreased rag1 expression in the thymus (white circle, D′), and defective facial and pharyngeal cartilage development (white arrow, alcian blue staining, ventral view, E′). These phenotypes are indicative of neural crest defects. (F, F′) Sema3D morphants have dysmorphic hearts with smaller ventricle (V), smaller atrium (A) and thickened myocardium (m). Endocardium was present (e), but AV valve and trabeculation were absent (4 dpf, lateral section, H&E stain, 100 μm scale bar). (G, G′) Magnified pictures of F and F′, respectively. Trabeculation (t) and AV valve formation (avv) observed in wild-type embryo was not found in morphant. In morphant, hypertrophic cardiomyocytes (hm) were observed. EXPRESSION / LABELING:
|
|
sema3D-splice blocking morpholino knocks down sema3D mRNA and phenocopies sema3D-augMO. (A) Genomic structure of sema3D gene (GenBank #NM 131048), exons (boxes, not to scale). Splice donor site between 4th intron and 5th exon (5th exon codes amino acids in sema domain) was targeted by sema3D-spMO. The red line indicates the major splice variant observed following spMO injection. Primers used for RT-PCR analysis in B are shown as arrows (forward 5′-CCA GAC AAC ATC AAT AAA CAC CCC-3′, reverse 5′-TTG CCC AGG AAA TCA GAC GC-3′). (B) The single fragment (exons 3–7, 350 bp) amplified from wild-type RNA was diminished by sema3D-spMO and replaced by a smaller fragment (arrow, 230 bp). Sequencing revealed that sema3D-spMO caused utilization of cryptic splice sites, deleting exon 5 and part of exon 6, removing amino acids 129 through 168 (nucleotides 656–775 in sema3D mRNA), deleting the essential sema domain. Sema3D-spMO morphants had the same phenotypes as sema3D-augMO, including severe pericardiac edema (C, arrow, 4 dpf), reduction of crestin expression in cranial neural crest (D, arrowheads, 8 SS), reduction of rag1 expression in the thymus (E, white circle, 4 dpf) and loss of facial and pharyngeal cartilages (F, Alcian blue staining, 4 dpf). EXPRESSION / LABELING:
|
|
Migratory defects were observed with foxd3 and AP2 in sema3D-aug morphants. In situ hybridization with foxd3 (A, A′, B, B′) and AP2 (C, C′, D, D′). Both markers were expressed including the cranial neural crest at 8 SS. Dorsal views (B, B′, D, D′, cranial up) showed that cells expressing both markers in morphants did not migrate as far as those in wild type, suggesting that migratory pattern was disrupted. Brackets in panels B, B′, D and D′ showed altered pattern of migration. White arrows in panels B, B′, D and D′ indicated the distance of migrating neural crest cells. EXPRESSION / LABELING:
|
|
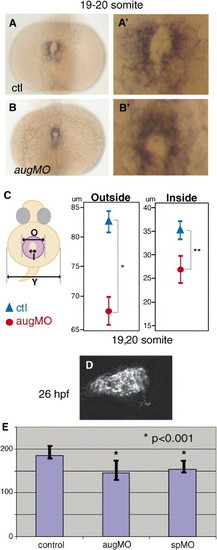
sema3D morphants have fewer cardiomyocytes in the primary heart field. Dorsal views cmlc2 expression in control (A, A′) and sema3D morphants (B, B′). (C) Schematic diagram of inside (I) and outside (O) diameters of cmlc2 expression (purple) and (Y) yolk diameter. Graphs indicate mean ± one standard deviation of diameters measured (μm) in sema3D morphants (red circles, n = 18) and controls (blue triangles, n = 13). The primary heart field is significantly smaller in sema3D morphants (*P = 0.0002, **P = 0.0549). (D) An image of dissociated cmlc2-gfp-positive heart at the 26 hpf, after arrival on neural crest. (E) Count of number of cardiomyocytes from dissociated cmlc2::EGFP embryos. y axis is mean number of EGFP-positive cardiomyocytes, bars indicate standard deviation. Control embryos had 184 ± 15 (n = 30) cardiomyocytes. Both aug-MO and sp-MO-injected embryos had significantly fewer GFP-positive cardiomyocytes (aug-MO: 145 ± 21, n = 23, sp-MO: 154 ± 11, n = 11) than control (*P < 0.0001). EXPRESSION / LABELING:
|
|
sema3D morphants have normal precardiomyocytes in LPM before neural crest arrival and diminished number of cardiomyocytes later. Dorsal views cmlc2 expression in control (A, A′) and sema3D-aug morphants (B, B′) at the 16 SS. Panels A′ and B′ are magnified cmlc2 expression pattern. (C) Schematic diagram of width (W) along the proximal–distal axis and height (H) along the anterior–posterior axis of cmlc2 expression (purple). Graphs indicate mean ± one standard deviation of width and height measured (μm) in sema3D morphants (red circles, n = 32) and controls (blue triangles, n = 37). Before the arrival of neural crest cells, the size of the primary heart field in sema3D morphants is similar to or slightly larger than the heart field in controls. *P = 0.624, **P = 0.09, ***P = 0.005. EXPRESSION / LABELING:
|
|
neuropilin1A is expressed in LPM and morphant phenotypes are similar to those of sema3D morphants. (A) At 8 SS, nrp1A RNA is strongly expressed in LPM (shown in arrowhead) and in the notochord (shown in arrow), not in neural crest. Dorsal view of embryo, cranial to the top. (B) rag1 expression in the thymus (white circle) was reduced in morphants. (C) Alcian blue staining showed defective facial and pharyngeal cartilage development (arrow, ventral view). (D) nrp1A morphants have dysmorphic hearts with smaller ventricle (V), smaller atrium (A) and thickened myocardium (m). Endocardium was present (e) (4 dpf, lateral section, H&E stain). EXPRESSION / LABELING:
|
|
|

Unillustrated author statements EXPRESSION / LABELING:
|
Reprinted from Developmental Biology, 298(1), Sato, M., Tsai, H.J., and Yost, H.J., Semaphorin3D regulates invasion of cardiac neural crest cells into the primary heart field, 12-21, Copyright (2006) with permission from Elsevier. Full text @ Dev. Biol.