- Title
-
poky/chuk/ikk1 is required for differentiation of the zebrafish embryonic epidermis
- Authors
- Fukazawa, C., Santiago, C., Park, K.M., Deery, W.J., Canny, S.G., Holterhoff, C.K., and Wagner, D.S.
- Source
- Full text @ Dev. Biol.
|
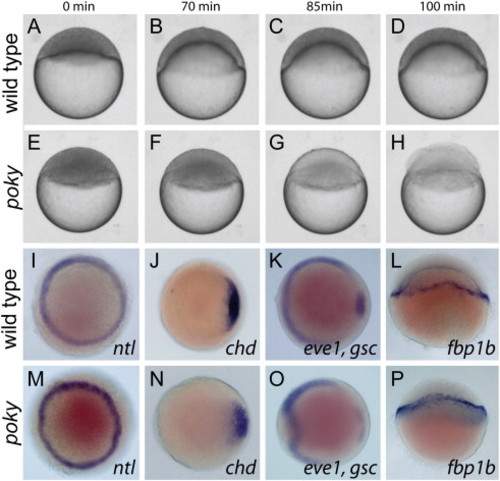
Poky mutants are delayed in epiboly. (A–H) Stills from time lapse analysis of epiboly in wild type (A–D) and poky mutant embryos (E–H) timed from sphere stage (0 min). (I–P) Whole mount in situ expression in 50% epiboly wild type (I–L) and age matched poky mutant (M–P) embryos, (I,M) ntl, (J,N) chd, (K,O) eve1, gsc, and (L,P) fbp1b. (A–H,L,P) Lateral views. (I–K,M–O) Animal pole views. EXPRESSION / LABELING:
PHENOTYPE:
|
|
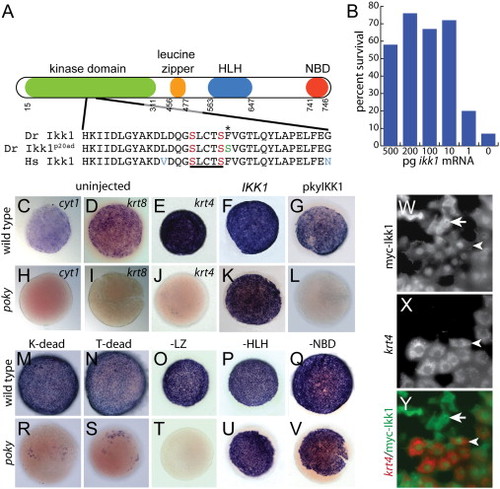
poky encodes chuk/ikk1/ikkα. (A) Schematic of domains of Ikk1. Amino acid sequence around the T-loop activation domain in wild type; poky mutant and human sequence is aligned below. The site of the missense mutation in pokyp20ad is indicated by the asterisk (*). T-loop activating serines are indicated in red. Missense mutation in pokyp20ad substituting the conserved phenylalanine for serine is indicated in green. Nonconserved amino acids in the human sequence are indicated in blue. (B) Percent rescue to 24 hpf by ikk1 mRNA. (C,D,E,H,I,J) Whole mount in situ hybridization for cyt1 (C,H), krt8 (D,I) and krt4 (E,J) at 50% epiboly. (F,G,K,L,M–V) whole mount in situ hybridization for krt4 at 50% epiboly following microinjection of the indicated mRNA. (W–Y) Cell autonomy of Ikk1. (W) Anti myc staining of myc-Ikk1 scatter labeled embryo. (X) krt4 expression of the same embryo. (Y) Merged image of W and X. Rescued EVL cells (arrowhead) and isolated myc positive DEL cells (arrow) can be observed. EXPRESSION / LABELING:
PHENOTYPE:
|
|
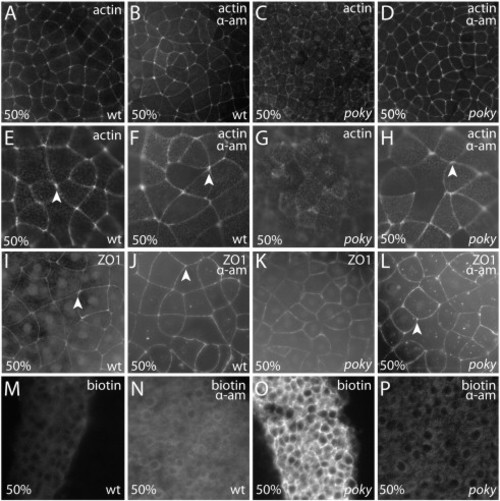
poky mutant embryos display junction protein localization and apical–basal polarity. Wild type embryos displayed tight localization of actin microfilaments at cell–cell boundaries (arrows) at sphere (A, magnified in C) and robust labeling of cell vertices at 50% epiboly (E magnified in G, arrowheads). poky mutant embryos displayed actin localization to cell–cell boundaries (arrows) at sphere stage (B magnified in D). At 50% epiboly (F magnified in H) the localization to cell–cell boundaries was less robust. Gaps were observed between neighboring cells (arrows) and little labeling was observed at cell vertices (arrowheads). Wild type embryos displayed localization of ZO1 to the tight junction at EVL cell borders at sphere (I magnified in K) and 50% epiboly (M magnified in O) with strong localization to cell–cell boundaries (arrows) and cell vertices (arrowheads). poky mutants also displayed localization at sphere stage (J, magnified in L). By 50% epiboly poky mutant cells were smaller but still displayed localized ZO1 (N magnified in P, arrow), although they lacked cell vertex labeling (arrowhead, N,P). Wild type (Q,S,U) and poky mutant (R,T,V) embryos display apical localization of aPKC (green, Q–T), localization of ZO1 to sites of EVL cell–cell contact (red, Q,R), basolateral localization of β-catenin (red, S,T) and cadherin (U,V). (W–Z) TEM of wild type (W,X) and poky mutant (Y,Z) EVL cells at 30% epiboly (tight junctions, black arrows). EXPRESSION / LABELING:
PHENOTYPE:
|
|
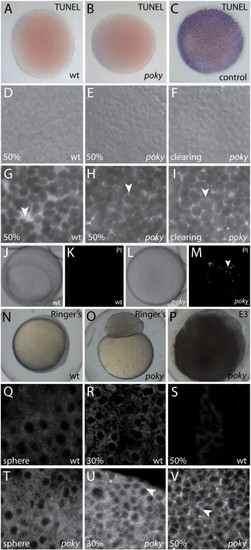
poky mutants fail to form an EVL barrier. Wild type (A), poky mutant (B) and wild type positive control (C) processed for TUNEL. DIC (D–F) and interstitial space labeled with fluorescent dextran (G–I) of wild type (D,G), intact poky mutants (E,H) and clearing poky mutants (F,I) reveal large spaces in the wild type embryo at 50% epiboly (arrowhead, G) but little interstitial space in poky mutants (arrowheads, H,I). White light (J,L) and fluorescent (K,M) of propidium iodide stained live wild type (J,K) and clearing poky mutants (L,M) shows labeling of multiple nuclei in poky mutants (arrowhead, M). Wild type control embryos at 100% epiboly develop normally in 1× Ringer′s saline (N). poky mutant embryo cultures in Ringer′s saline did not lyse but failed to initiate epiboly (O). poky mutant embryos cultured in E3 displayed blastoderm lysis when controls reached 50% epiboly (P). Wild type embryos (Q–S) displayed little interstitial biotin labeling. poky mutant embryos showed little interstitial label at sphere stage (T). 30% epiboly poky mutant embryos had some interstitial signal (arrowhead, U). At 50% epiboly poky mutants had strong interstitial label throughout the blastoderm (arrowhead, V). PHENOTYPE:
|
|
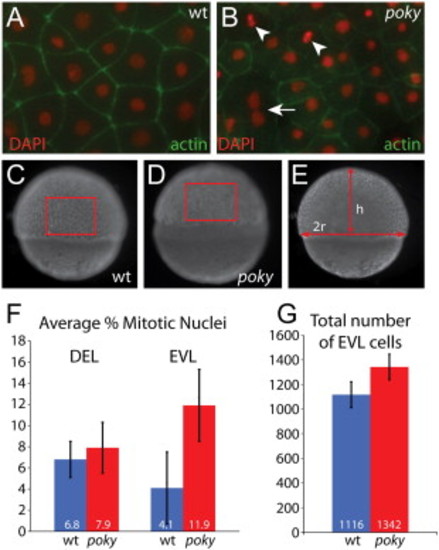
poky mutants fail to down regulate cell division in the EVL. Embryos were stained with phalloidin (green) to reveal actin localization at cell boundaries and DAPI (red) to reveal nuclear morphology. Dividing cells were identified by their nuclear morphology (arrowheads, A,B). Multinucleate EVL cells were observed in wild type and mutant embryos (arrow, B). Total number of EVL cells was calculated by determining the number of cells per unit area as C and D and then calculating the total EVL surface area by calculating the radius and height as in E. (F) Percent mitotic nuclei. (G) Total number of EVL cells PHENOTYPE:
|
|
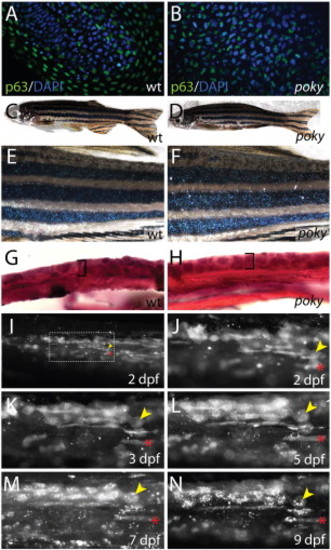
poky mutant adults have normal skin and wild type EVL cells persist into larval stages. (A,B) Optical section through wild type (A) and poky mutant (B) epidermis expressing nuclear p63 protein (green) at 24 hpf. (C,D) 2 year-old wild type and poky mutants have no obvious skin lesions. (E,F) Pigment, scales and fins are normal in wild type (E) and poky mutants (F). Histology of adult skin reveals the typical bilayered epidermis (bracket) in wild type (G) and poky mutants (H). Lineage tracing of embryonic and larval EVL (I–N). EVL from embryos scatter labeled with fluorescent dextran was observed up to 9 dpf. (I) 2 dpf low magnification image of the tail shows multiple cells labeled; location at higher magnification in J. (J) A clone of EVL cells is easily observable at 2 dpf (yellow arrowhead marks a single EVL cell). The location of these cells was traced relative to underlying muscle cells (red asterisk) at 2 dpf (J), 3 dpf (K), 5 dpf (L), 7 dpf (M) and 9 dpf (N). |
|
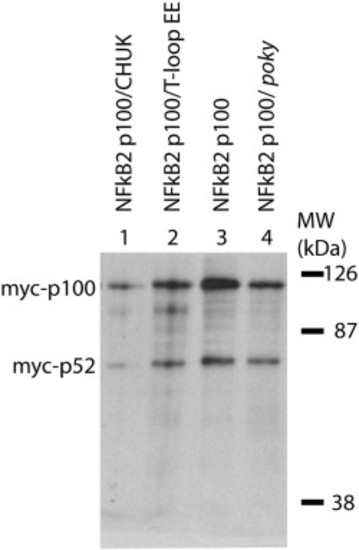
Poky/IKK1 is not required for constitutive processing of NFkB2 in the early zebrafish embryo. Expression of mRNA encoding myc-NFkB2 (p100) was detected by anti-myc western blot analysis in wild type (lane 3), poky mutant (lane 4), wild type expressing IKK1 (lane 1) and wild type expressing a T-loop phosphomimetic substitution of Ikk1 (lane 2). In all conditions p100 and processed p52 were observed. |
Reprinted from Developmental Biology, 346(2), Fukazawa, C., Santiago, C., Park, K.M., Deery, W.J., Canny, S.G., Holterhoff, C.K., and Wagner, D.S., poky/chuk/ikk1 is required for differentiation of the zebrafish embryonic epidermis, 272-283, Copyright (2010) with permission from Elsevier. Full text @ Dev. Biol.